11 June 2021: Original Paper
Sequential Organ Failure Assessment (SOFA) Score-Based Factors Predict Early Mortality in High-Risk Patients with Living Donor Liver Transplant
Hao-Chien Hung1ABC, Chin-Hsin Shen2ABC, Chen-Fang Lee13ABCDEF*, Ssu-Min Cheng4C, Wei-Chen Lee13ADOI: 10.12659/AOT.931045
Ann Transplant 2021; 26:e931045
Abstract
BACKGROUND: Patients with a Sequential Organ Failure Assessment (SOFA) score >7 on post-transplant day (POD) 7 have been reported to have a higher risk of short-term mortality after living donor liver transplant (LDLT). We sought to identify factors that were associated with early mortality in this high-risk population.
MATERIAL AND METHODS: A total of 102 patients with a high SOFA score (>7) on POD 7 were enrolled, of which 72 (70.6%) were assigned to the survivor group, and the other 30 (29.4%) patients were assigned to the non-survivor group according to post-transplant 3-month results. Demographics, clinical data, operative parameters, and individual SOFA component scores were collected. Independent risk factors for 3-month mortality were identified by multivariate logistic regression analysis using backward elimination procedures.
RESULTS: Of 102 high SOFA score patients, the 3-month mortality rate after LDLT in our study was 29.4%. Four independent risk factors were indicative for early death: graft-to-recipient weight ratio (GRWR) <0.8 (hazard ratio [HR]=3.00; 95% CI=1.05-8.09; P=0.041), longer warm ischemia time (HR=37.84; 95% CI=1.63-880.77; P=0.024), high liver component of the SOFA score, and cardiovascular component of the SOFA score (liver component: HR=10.39; 95% CI=1.77-60.89; P=0.009 and cardiovascular component: HR=13.34; 95% CI=2.22-80.12; P=0.005).
CONCLUSIONS: In conclusion, 3-month mortality among patients with high SOFA score on POD 7 is associated with multiple independent risk factors, including smaller GRWR, longer warm ischemia time, and higher category of liver and cardiovascular component of SOFA score. By recognizing high-risk patients earlier, the LDLT outcomes may be improved by timely intensive therapies.
Keywords: Liver Transplantation, Risk Factors, End stage liver disease, Living Donors, Organ Dysfunction Scores, Severity of Illness Index
Background
Liver transplantation (LT) remains the optimal treatment for patients with advanced chronic liver disease, acute decompensated liver failure, and hepatocellular carcinoma [1]. Despite great improvement in surgical techniques and progress in managing allograft and critical care [2,3], early vital organs failure remains a major obstacle while managing transplant patients.
Several studies had searched for possible risk factors affecting early mortality after LT [4,5]. However, most of these studies focused on the entire population rather than a particular group with extremely high risk.
The Sequential Organ Failure Assessment (SOFA) [6] score was initially developed to describe and detect organ failure in sepsis settings and has become one of the most comprehensive tools to predict early outcomes in various intensive care units (ICU) [7,8]. The SOFA score [6] was traditionally calculated by a sum of corresponding scores from 6 components – respiratory, coagulation, cardiovascular, liver, renal, and central nervous system (CNS) – with a 0 to 4 scale, shown in Supplementary Table 1. Our group previously reported that patients with a SOFA [6] score >7 on post-transplant day 7 are considered to have a higher risk of short-term mortality after LT [9]. The 3-month mortality rate in this high-risk population was found to be up to 67.5% [7]. This conclusion has been examined to discriminate between survivors and non-survivors [10]. Nevertheless, some patients with high post-transplant SOFA scores still can recover from a critical condition, and the most important factors remains inconclusive.
In this study we tried to validate the SOFA model for a larger cohort in the last 10 years. We aimed to identify decisive factors associated with mortality in the high-risk population of living donor liver transplantation (LDLT) to improve the overall survival.
Material and Methods
STUDY POPULATION AND DATA COLLECTION:
A total of 527 consecutive LDLT cases, during the period between January 2010 and March 2019, were retrospectively reviewed. Exclusion criteria were: pediatric patients (<18 years old, n=3), lack of essential SOFA information (n=5), and post-transplant day 7 SOFA score ≤7 (n=417). Subsequently, we enrolled 102 patients with high POD 7 SOFA scores (>7). This study was approved by our Institutional Review Board (No. 202001325B0). All patients fit the standard indication for the necessity of liver transplantation. Associated clinical information, laboratory data, and SOFA scores on POD 7 were documented. Preoperative data included recipient and donor age, sex, body mass index, ABO compatibility between the recipient and the donor, model for end-stage liver disease (MELD) score, Child-Pugh class, primary etiology of liver disease, and relevant medical history. We also collected intraoperative data on ischemia time, total operation time, ascites amount, blood loss, and graft-to -recipient weight ratio (GRWR). The survival period was defined as the time from liver transplant until death due to any causes and was censored on the last follow-up date on which the patient was alive. Our primary outcome was post-transplant 3-month survival, and we further divided all patients into survivor (n=72) and non-survivor groups (n=30).
SOFA SCORE CALCULATION AND SOFA COMPONENTS DICHOTOMY:
We simplified these SOFA categories by dichotomizing each organ system score by an optimal cut-off value, which was determined by receiver operating characteristic curve to predict post-transplant 3-month mortality. Each SOFA category was dichotomized by cut-off values: 0 for cardiovascular, 2 for coagulation, 1 for renal, 2 for liver, 2 for respiration, and 0 for CNS.
LDLT PROGRAMS AND POST-TRANSPLANT CARE:
Before getting approval for performing a liver transplant, routine work-up must be completed, including liver and vascular images, echocardiogram, pulmonary function test, a variety of urine and blood tests (to determine blood type, clotting function, biochemical studies, and serology screening), and consultations with the transplant team members (hepatologist, surgeon, psychologist, transplant coordinator, social worker, and other specialists, as dictated by particular needs). In addition, patients need to receive pre-liver transplant HLA compatibility screening and lymphocyte cross-matching, and a goal of anti-blood type isoagglutinin titers equal to or less than 1: 64 is desired while performing ABO-incompatible LDLT. Two different pre-transplant rituximab regimens are given according to the anti-A and -B isoagglutinin titers before transplantation, and extra plasmapheresis or plasma exchange was given for patients who failed to reach the goal before receiving transplantation. The details and results of our pre-transplant preparation and post-transplant immunosuppression were illustrated in a previously published article [11].
We performed LDLT using standard technique, as our previous publications described [12,13], and we do not routinely remove the spleen. Regarding prophylactic antibiotics, our routine program suggests a third-generation cephalosporin prescription for uncomplicated patients with a lower MELD score (<20). For patients with a threshold MELD score greater than 20 or who have a complex medical condition (eg, tense ascites, older recipient age, massive intraoperative blood loss), the combination of carbapenem, vancomycin, and echinocandins is administrated [14].
Regarding post-transplant care, ICU care is indispensable to observe and check the progress of vitals and graft condition. The length of ICU stay varies according to individual’s medical stability, and it usually ranges from 5 to 10 days. An extension of ICU stay is indispensable when a patient has organ failure in one or more organs in the first week, including: neurologic or respiratory failure requiring ventilator dependence, persistent shock status, acute deterioration of kidney function causing fluid/electrolyte imbalance requiring renal replacement therapy, uncontrolled or new bleeding episode, any medical emergency that needs a second operation, or any combination of these factors.
STATISTICS:
The independent
Results
CHARACTERISTICS OF ENROLLED PATIENTS:
Table 1 shows recipient and donor characteristics, surgery-related factors, and total SOFA score and corresponding components on POD 7 of 102 high-risk LDLT cases. The mean age was 53.3±9.5 and 33.1±9.2 years for recipients and donors, respectively. Most of the recipients were male (n=73, 71.6%), while the sex ratio of donors was about equal (n=47, 46.1%). The leading etiology was viral hepatitis (hepatitis B virus: n=65, 63.7%; hepatitis C virus: n=30, 29.4%), and 22 (21.6%) patients had hepatocellular carcinoma. There were 39 (38.2%) patients with a history of alcohol abuse. The mean MELD score was 24.9±10.5 (median: 24, range: 8–40). Most of our liver grafts were right livers, with a mean GRWR of 0.95±0.24 (median: 0.92, range: 0.54–1.74). The mean cold ischemia time, warm ischemia time, and total procedure time were 45.8±44.6, 37.1±9.6, and 653.5±111 minutes, respectively. Concerning the SOFA score on POD 7, a mean total score of all 102 included patients was 9.8±1.9 (median: 9, range: 8–16). The distribution of case numbers was concentrated at SOFA scores 8 and 9 (n=58/102, 56.9%) among enrolled patients (Figure 1). Of this entire cohort, without regard to high or low SOFA score on POD 7 (n=519), the post-transplantation 3-month survival rate was 91.9%. On the contrary, the 3-month survival rate after transplant was 97.1% for patients with low SOFA (≤7, n=417) and 70.6% for high SOFA score (>7, n=102; p<0.001, Figure 2).
COMPARISON BETWEEN SURVIVORS AND NON-SURVIVORS:
There were 102 patients with a high total SOFA scores (>7) on POD 7 enrolled, of which 72 (70.6%) patients were classified to the survivor group, and the other 30 (29.4%) patients were in the non-survivor group according to post-transplant 3-month results. A comparison between the 2 groups is summarized in Table 2. The prevalence of liver disease etiology, severity of liver disease, and MELD score did not significantly differ between the 2 groups. However, the proportion of patients with older donor age (>45 years old), smaller GRWR (<0.8%), and longer warm ischemia time (>60 minutes) was significantly higher in the non-survivor group. Compared to patients in the survivor group, non-survivors tended to have a higher total SOFA score on POD 7 (10.8±2.2 vs 9.3±1.5, P<0.001), and a higher category in cardiovascular and liver components was associated with early mortality after transplant (both P<0.05).
Complications are also shown in Table 2. Significant differences between the 2 groups were found, and there was a higher proportion of infection (50.0% vs 29.2%, p=0.045), rejection (23.3% v.s 6.9%, p=0.019), and vascular complications (13.3% v.s 0.0%, P=0.002) in the non-survivor group. Furthermore, ventilator-dependence (53.3% vs 26.4%, P=0.009), either failure of weaning within 48 hours after transplant or re-intubation of endotracheal tube within 7 days, seemed to be more frequent in the non-survivor group.
Among the survivors, 2 of 3 patients (n=48/72, 66.7%) had a concentrated SOFA score between 8 and 9 on POD 7 (Figure 3A). The composition of case numbers was dispersed among individual SOFA scores in the non-survivor group (Figure 3B).
INDEPENDENT RISK FACTORS FOR 3-MONTH MORTALITY IN PATIENTS WITH HIGH POD 7 SOFA SCORES:
In univariate analysis, we analyzed all available clinical factors, and 5 factors (older donor age, smaller GRWR, longer warm ischemia time, and higher liver and cardiovascular component) were subsequently considered as potential risk factors (P<0.100) associated with 3-month mortality after transplant, as shown in Table 3. These potential risk factors were entered into multivariate analysis, showing that GRWR <0.8 (hazard ratio [HR]=3.00; 95% confidence interval [CI]=1.05–8.09; P=0.041) and higher categorized liver component of SOFA score (HR=10.39; 95% CI=1.77–60.89; P=0.009), cardiovascular component of SOFA score (HR=13.34; 95% CI=2.22–80.12; P=0.005), and longer warm ischemia time (HR=37.84; 95% CI=1.63–880.77; P=0.024) were independent risk factors for 3-month mortality after LDLT. We compared Kaplan-Meier survival curves by numbers of risk factors a patient had (21 patients had 0 risk factors, 52 patients had 1 risk factor, 26 patients had 2 risk factors, and 3 patients had 3 risk factors; none of them had all 4 risk factors), and the group with multiple risk factors had inferior outcomes (P<0.001, Figure 4).
THE ASSOCIATION BETWEEN HIGH SOFA COMPONENTS AND ADVERSE EVENTS:
It is relatively common to have a high-risk liver component score (>2); for total serum bilirubin > 6 mg/dL, there were 72 cases among that fulfilled the criterion. After excluding patients who were actually having a fair recovery from pre-transplant hyperbilirubinemia (n=30), the remaining 42 patients experienced a variety of conditions that deteriorated their liver function. Most had various degrees of infection (n=21), and 6 of them were directly associated with biliary complications, and 2 were cytomegalovirus disease-related. Acute rejection occurred in 11, and more than half (n=6) eventually lost the graft. Vascular complications (n=4; 3 insufficient portal inflow and 1 hepatic venous outflow obstruction), cardiopulmonary complications (n=3; 2 acute myocardial infarctions and 1 cardiac arrhythmia), and massive hemorrhagic events (n=3) were also responsible for high liver component score (>2) on POD 7.
On the other hand, there were 11 cases with high-risk cardiovascular component scores (>0), such as hypotension with a mean arterial pressure less than 70 mmHg, and only 1 of them was directly heart-originated because of acute myocardial infarction. The rest of them were mostly due to septic shock (n=8), followed by hemorrhagic shock (n=1), and portal vein thrombosis-related graft and multiple organ failures (n=1).
CAUSES OF DEATH IN THE HIGH-RISK POPULATION:
The causes of death are summarized in Table 4. Of the 30 deaths within 90 days after liver transplantation, 7 (23.3%) deaths were considered to be related to rejection, with the majority owing to a combination of either simultaneous or subsequent infection. A primary critical infection accounted for 15 deaths (50.0%): 8 originated from the respiratory tract, 4 were due to intraabdominal infection, 1 was associated with urinary sepsis, 1 was attributed to central nervous system infection, and the last 1 was due to catheter-associated blood stream infection. Other less common causes of death were vascular complications, either portal inflow insufficiency or hepatic venous outflow obstruction (n=4, 13.3%), cardiopulmonary failure (n=2, 6.7%), and bleeding (n=2, 6.7%). Table 4 also reveals the relationship between numbers of independent risk factors (0 to 3) that individuals had and the causes of death. Although rejection and vascular complications seemed to have more risks, there was no strong correlation among SOFA scores, causes of death, and the number of independent risks.
Discussion
In the current study targeting patients with high POD 7 SOFA score (>7), already considered to be a high-risk population, we found that smaller GRWR, longer warm ischemia time, and higher category of cardiovascular and liver component of SOFA score were correlated to post-transplant short-term adverse outcomes. Interestingly, 2 factors (GRWR and warm ischemia time) outweighed some SOFA components on POD 7 except for cardiovascular and liver component of SOFA score. They can be accessed beforehand to make medical adjustment and prepare in advance for those who might be at very high risk of short-term mortality. In liver allograft recipients, it is important to predict their outcomes. The SOFA scoring scale is an objective assessment to evaluate vital system function, especially in critically ill patients [7]. A convention of GRWR <0.8% has been generally considered as a poor indicator for post-transplant liver function, and it can compromise LT outcomes [15,16]. It is evident that the length of warm ischemia time should be controlled to within 60 minutes to reduce post-transplant complications, especially acute kidney injury [17].
Several SOFA-based prediction models [9,10,18] displayed high discrimination power in predicting mortality after LT, but none paid particular attention to SOFA components and their impact or focused on these sicker patients. We believe that the essence of the SOFA score after LT is the liver component, which is also an indispensable criterion for early allograft dysfunction diagnosis [19]. Moreover, most of our 3-month mortalities were complicated with an infectious condition, which may play a role in predisposing to liver graft dysfunction or be a result of graft failure. On the other hand, the cardiovascular component of the SOFA score is a clinical indicator of circulatory function and severity of sepsis, and reflects treatment response. It is a common problem in dealing with early post-transplant infection [20,21], especially when trying to reduce the risk of rejection by increasing immunosuppressive therapy. When the inflammatory reaction is compromised by anti-rejection drugs, the microbial invasion is often in the process of spreading when revealed clinically [22]. Therefore, a thorough routine examination of patients to identify obscure infectious sources and give early interventions, such as lower immunosuppressive level and apply broad-spectrum antibiotics, is fundamental.
Elsayed et al demonstrated that the duration of ICU stay is also a significant risk factor related to early mortality after living donor liver transplantation [23]. However, in the current study, the ICU stay length between the 2 groups did not differ significantly. A possible explanation might be the relatively short observation period (90 days) and high-risk patients (SOFA score >7 on POD 7) we assessed. An extension of ICU stay was common in the population we chose (mean ICU stay: 28.2 days in the current study vs 9.5 in Elsayed et al), which had a trend of needing more medical care and frequent observation of vital and graft conditions.
Our study found that patients with multiple risk factors tend to have an inferior prognosis. Therefore, efforts are started in donor selection to avoid grafts with small GRWR and preclude complex biliary anastomoses if possible. Detailed and precise surgical planning and execution are needed to avoid unnecessary risks and long ischemia time. In post-transplant critical care, the first step is to identify the subgroup of patients who are at high risk of developing adverse outcomes in the early post-operative period. Preventing and early detection of acute allograft rejection is a major issue to address, especially in patients with infectious concerns. We believe that the adoption of a consummate LDLT protocol and reducing associated risks will help to improve transplant outcomes.
This study has several limitations. First, the retrospective nature restricted the ability to access a serial measure and to evaluate whether the reversal of organ failure helps to improve patient outcomes. Second, this research was conducted in a single tertiary medical center, and conflicts may arise from possible protocol bias between our facility and other facilities. Larger and prospective studies are required to confirm our findings.
Conclusions
In conclusion, 3-month mortality among patients with high SOFA score on POD 7 is associated with multiple independent risk factors, including smaller GRWR, longer warm ischemia time, and higher category of cardiovascular and liver component of SOFA score. By reducing risk factors in high-risk populations, the LDLT outcomes can make greater progress.
Figures
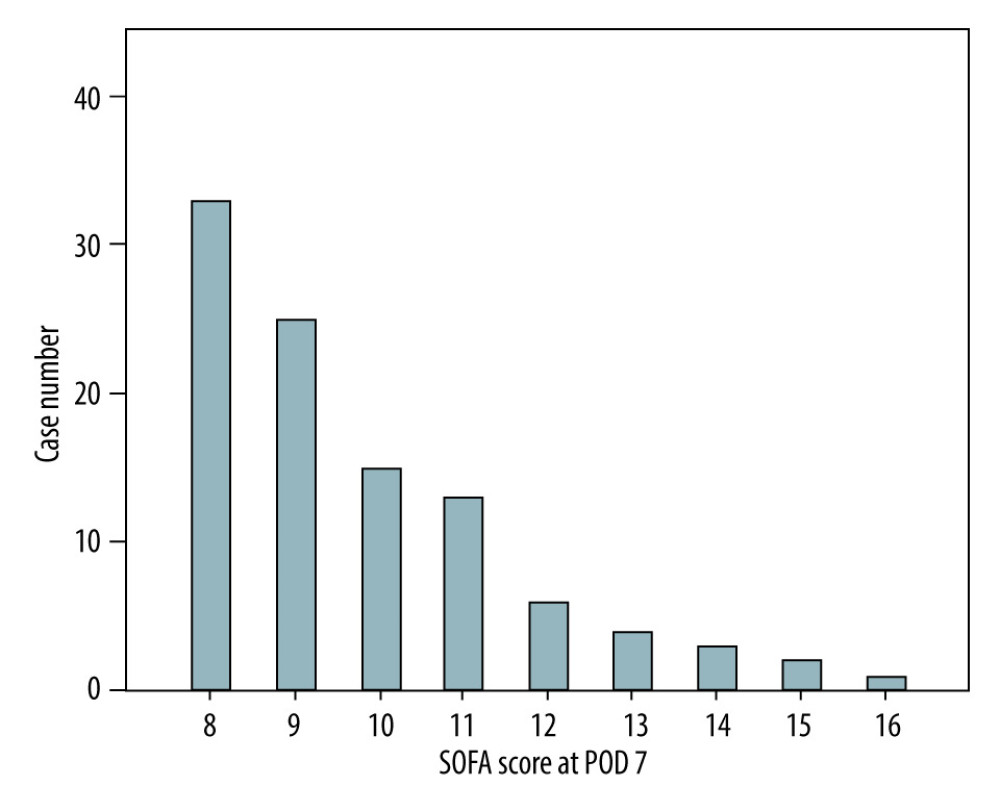 Figure 1. Distribution of SOFA scores on POD 7 among the enrolled population (n=102) in the current study.
Figure 1. Distribution of SOFA scores on POD 7 among the enrolled population (n=102) in the current study. 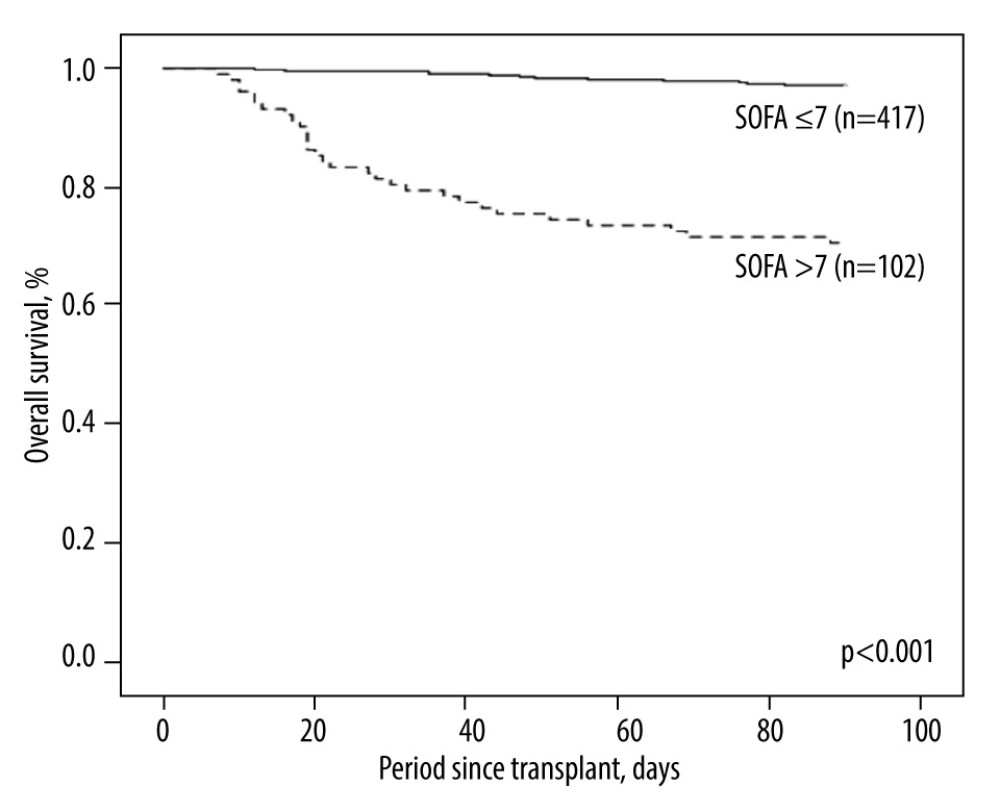 Figure 2. Kaplan-Meier plot of the 3-month survival according to high and low SOFA score on POD 7 with a cut-off value of 7. There was significant survival difference (P<0.001) between the 2 groups.
Figure 2. Kaplan-Meier plot of the 3-month survival according to high and low SOFA score on POD 7 with a cut-off value of 7. There was significant survival difference (P<0.001) between the 2 groups. 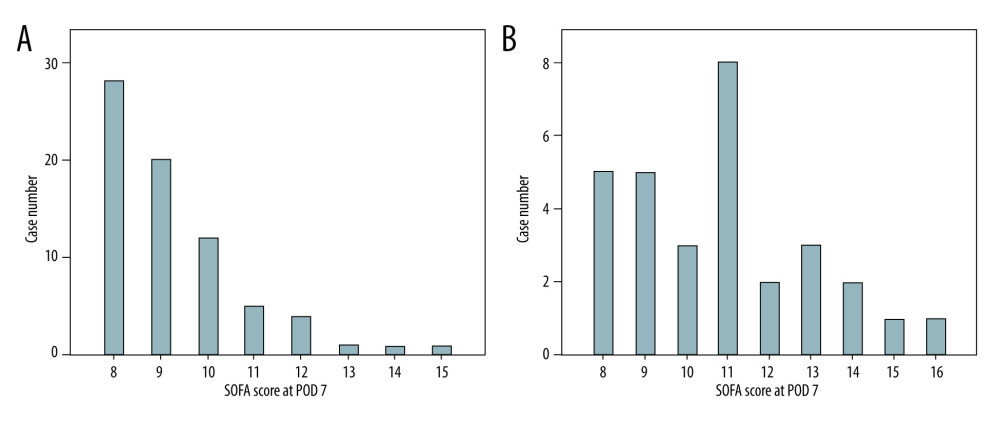 Figure 3. Distributions of SOFA score on POD 7 for the survivor group (n=72; A) and the non-survivor group (n=30; B). The case numbers decreased as the SOFA score increased in the survivor group but was dispersed in the non-survivor group.
Figure 3. Distributions of SOFA score on POD 7 for the survivor group (n=72; A) and the non-survivor group (n=30; B). The case numbers decreased as the SOFA score increased in the survivor group but was dispersed in the non-survivor group. 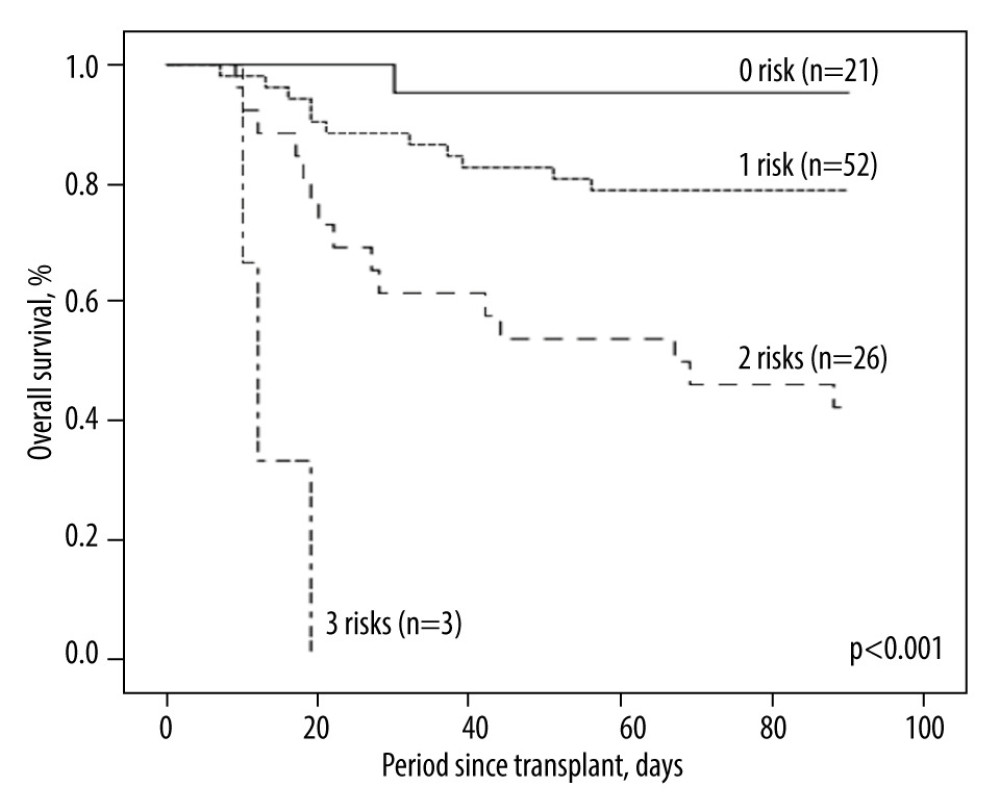 Figure 4. Kaplan-Meier plot of post-transplant 3-month survival according to the number of independent risks. The more risks the patients carried, the higher mortality rates they would have.
Figure 4. Kaplan-Meier plot of post-transplant 3-month survival according to the number of independent risks. The more risks the patients carried, the higher mortality rates they would have. Tables
Table 1. Demographic characteristics of 102 patients with post-transplant day 7 SOFA score >7.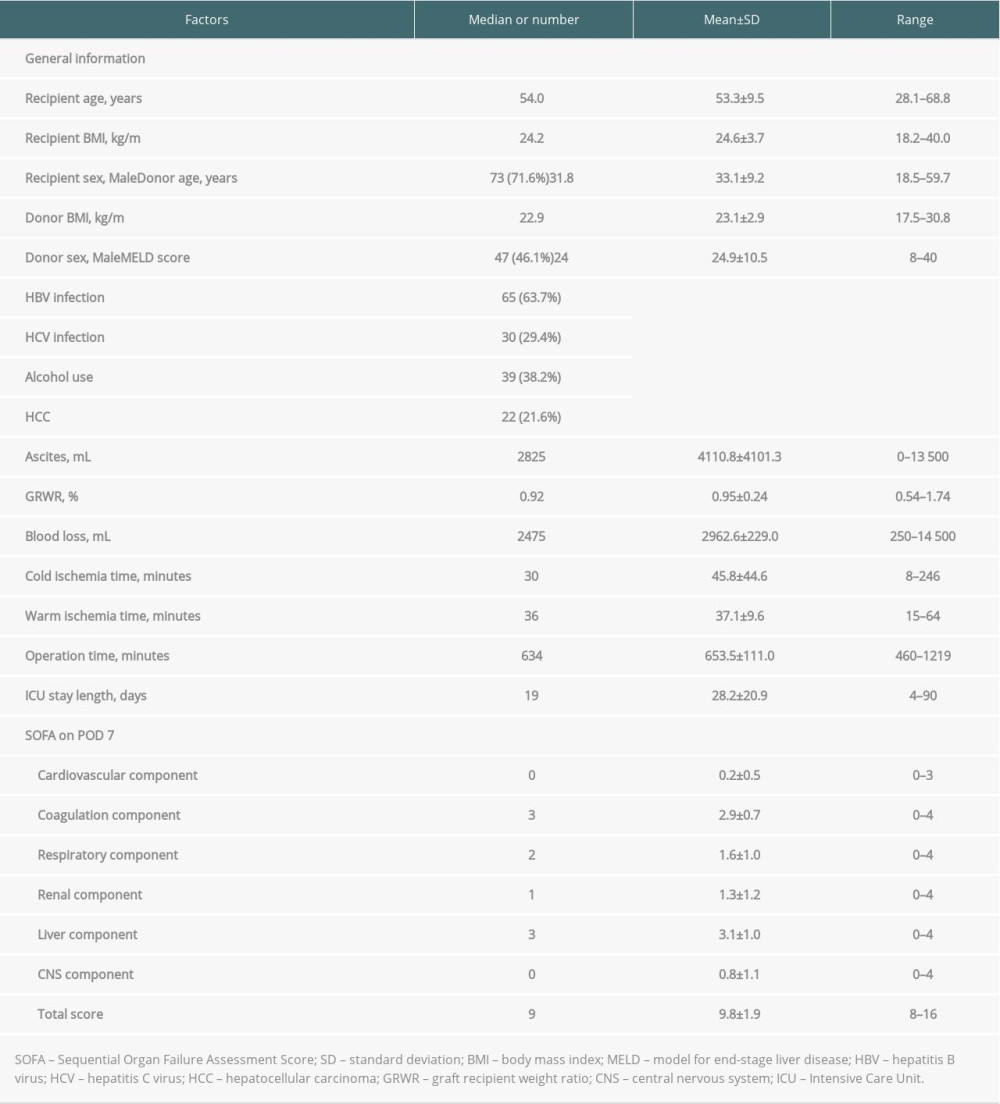 Table 2. Baseline demographics and clinical characteristics of high SOFA score patients by 3-month mortality.
Table 2. Baseline demographics and clinical characteristics of high SOFA score patients by 3-month mortality.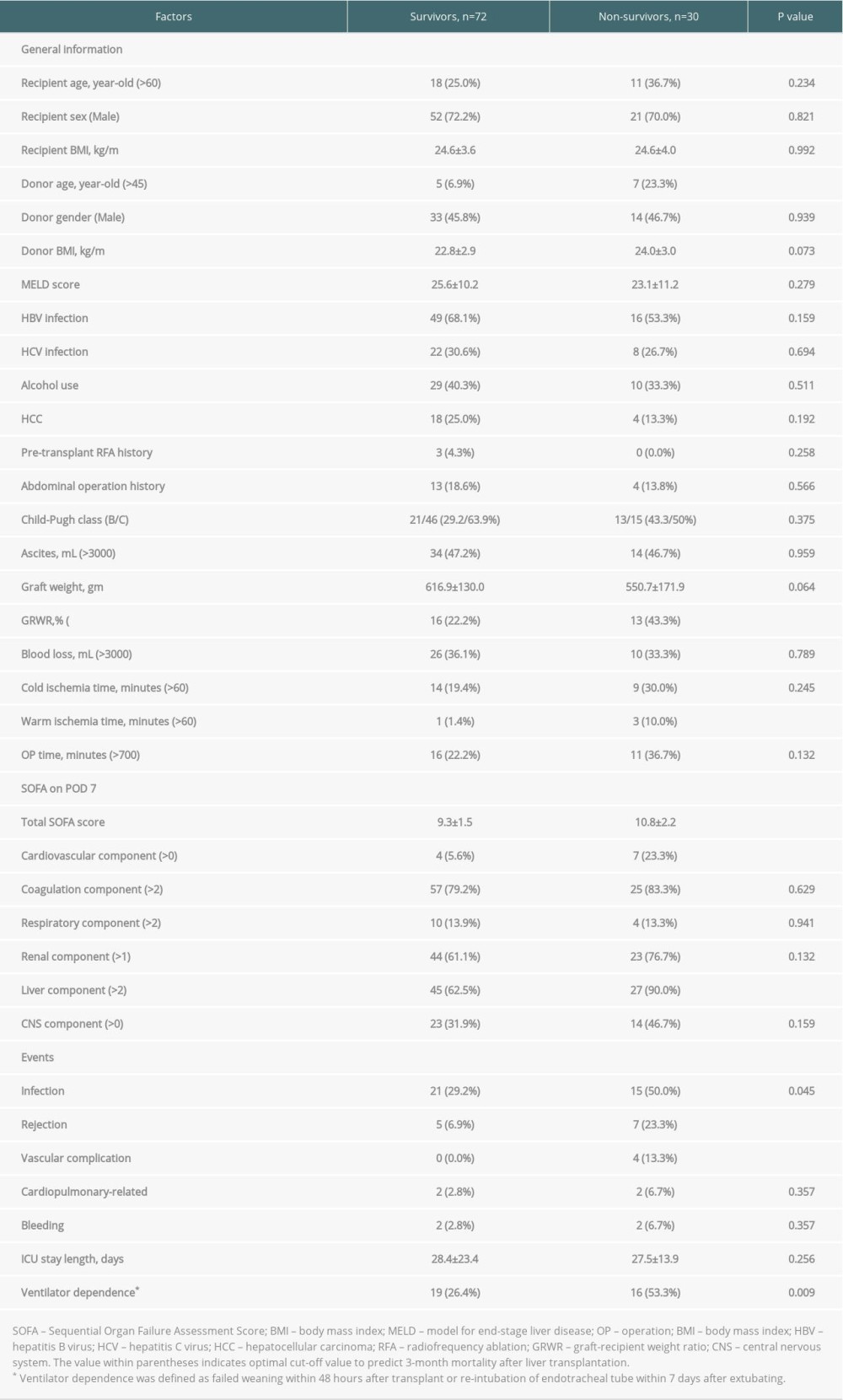 Table 3. Uni-/multivariate analyses of clinical characteristics on the 3-month mortality in high POD 7 SOFA score patients by logistic regression.
Table 3. Uni-/multivariate analyses of clinical characteristics on the 3-month mortality in high POD 7 SOFA score patients by logistic regression.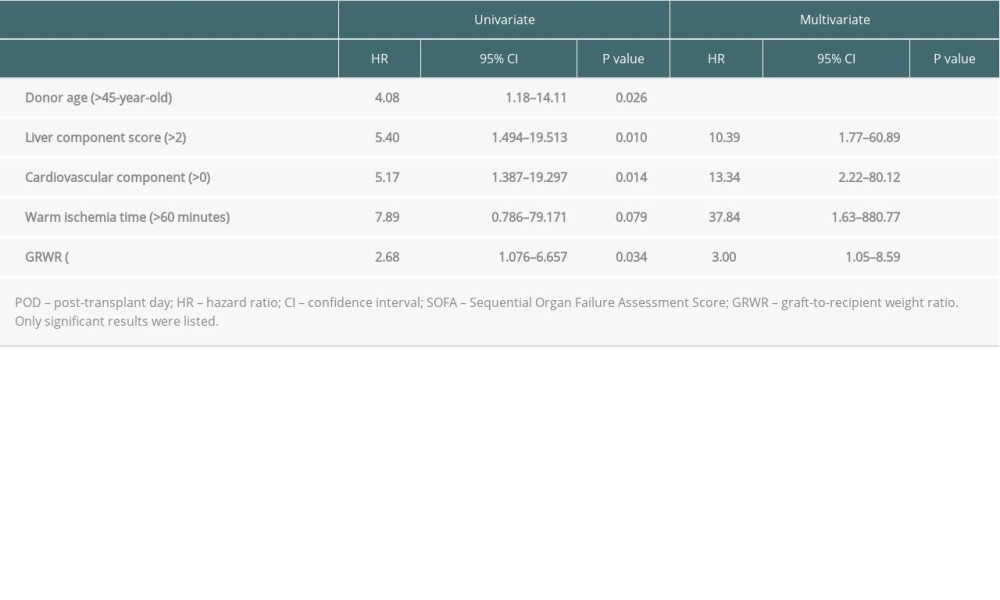 Table 4. Causes of death among non-survivors according to numbers of independent risks contained.
Table 4. Causes of death among non-survivors according to numbers of independent risks contained.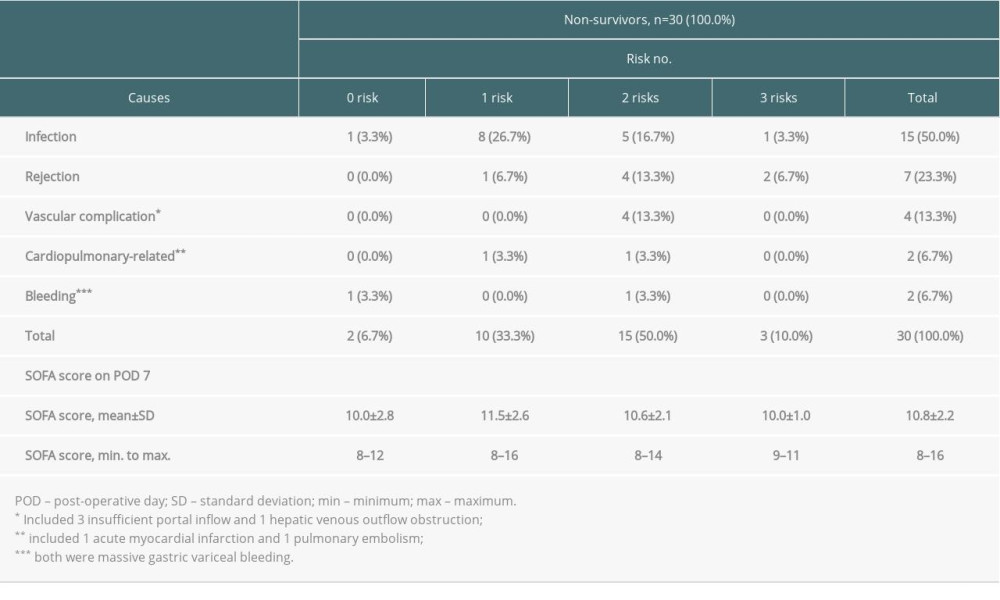 Supplementary Table 1. The sequential organ failure assessment (SOFA) score and its components.
Supplementary Table 1. The sequential organ failure assessment (SOFA) score and its components.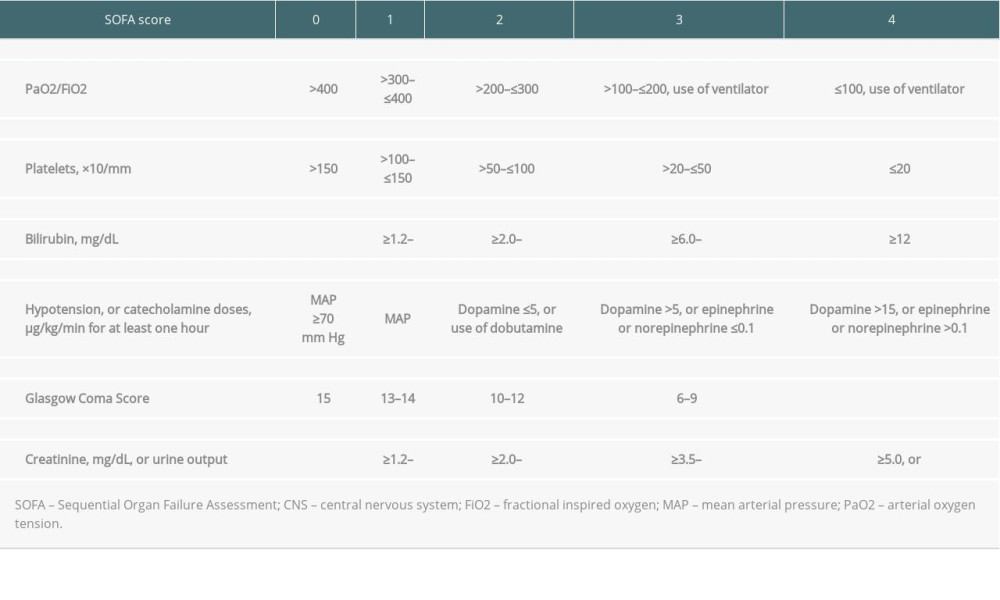
References
1. Zarrinpar A, Busuttil RW, Liver transplantation: Past, present and future: Nat Rev Gastroenterol Hepatol, 2013; 10(7); 434-40
2. Park GC, Song GW, Moon DB, Lee SG, A review of current status of living donor liver transplantation: Hepatobiliary Surg Nutr, 2016; 5(2); 107-17
3. Pillai VG, Chen CL, Living donor liver transplantation in Taiwan-challenges beyond surgery: Hepatobiliary Surg Nutr, 2016; 5(2); 145-50
4. Qian YB, Cheng GH, Huang JF, Multivariate regression analysis on early mortality after orthotopic liver transplantation: World J Gastroenterol, 2002; 8(1); 128-30
5. Murray KF, Carithers RL, AASLD practice guidelines: Evaluation of the patient for liver transplantation: Hepatology, 2005; 41(6); 1407-32
6. Vincent JL, Moreno R, Takala JOn behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine, The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure: Intensive Care Med, 1996; 22(7); 707-10
7. Ferreira FL, Bota DP, Bross A, Serial evaluation of the SOFA score to predict outcome in critically ill patients: JAMA, 2001; 286(14); 1754-58
8. Minne L, Abu-Hanna A, de Jonge E, Evaluation of SOFA-based models for predicting mortality in the ICU: A systematic review: Crit Care, 2008; 12(6); R161
9. Wong CS, Lee WC, Jenq CC, Scoring short-term mortality after liver transplantation: Liver Transpl, 2010; 16(2); 138-46
10. Pan HC, Jenq CC, Lee WC, Scoring systems for predicting mortality after liver transplantation: PLoS One, 2014; 9(9); e107138
11. Lee CF, Cheng CH, Wang YC, Adult living donor liver transplantation across ABO-incompatibility: Medicine (Baltimore), 2015; 94(42); e1796
12. Wu TJ, Dahiya D, Lee CS, Impact of portal venous hemodynamics on indices of liver function and graft regeneration after right lobe living donor liver transplantation: Liver Transpl, 2011; 17(9); 1035-45
13. Chan KM, Cheng CH, Wu TH, Clinical strategy for the reconstruction of middle hepatic vein tributaries in right liver living donor liver transplantation: World J Surg, 2014; 38(11); 2927-33
14. Chen YC, Huang TS, Wang YC, Effect of prophylactic antifungal protocols on the prognosis of liver transplantation: A propensity score matching and multistate model approach: Biomed Res Int, 2016; 2016 6212503
15. Ito T, Kiuchi T, Yamamoto H, Changes in portal venous pressure in the early phase after living donor liver transplantation: Pathogenesis and clinical implications: Transplantation, 2003; 75(8); 1313-17
16. Ben-Haim M, Emre S, Fishbein TM, Critical graft size in adult-to-adult living donor liver transplantation: Impact of the recipient’s disease: Liver Transpl, 2001; 7(11); 948-53
17. Kalisvaart M, Schlegel A, Umbro I, The impact of combined warm ischemia time on development of acute kidney injury in donation after circulatory death liver transplantation: Stay within the golden hour: Transplantation, 2018; 102(5); 783-93
18. Michard B, Artzner T, Lebas B, Liver transplantation in critically ill patients: Preoperative predictive factors of post-transplant mortality to avoid futility: Clin Transplant, 2017; 31(12); 13115
19. Olthoff KM, Kulik L, Samstein B, Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors: Liver Transpl, 2010; 16(8); 943-49
20. Sun HY, Cacciarelli TV, Singh N, Identifying a targeted population at high risk for infections after liver transplantation in the MELD era: Clin Transplant, 2011; 25(3); 420-25
21. Kim SI, Bacterial infection after liver transplantation: World J Gastroenterol, 2014; 20(20); 6211-20
22. Sawyer RG, Crabtree TD, Gleason TG, Impact of solid organ transplantation and immunosuppression on fever, leukocytosis, and physiologic response during bacterial and fungal infections: Clin Transplant, 1999; 13; 260-65
23. Elsayed FG, Sholkamy AA, Elshazli M, Comparison of different scoring systems in predicting short-term mortality after liver transplantation: Transplant Proc, 2015; 47(4); 1207-10
Figures
 Figure 1. Distribution of SOFA scores on POD 7 among the enrolled population (n=102) in the current study.
Figure 1. Distribution of SOFA scores on POD 7 among the enrolled population (n=102) in the current study. Figure 2. Kaplan-Meier plot of the 3-month survival according to high and low SOFA score on POD 7 with a cut-off value of 7. There was significant survival difference (P<0.001) between the 2 groups.
Figure 2. Kaplan-Meier plot of the 3-month survival according to high and low SOFA score on POD 7 with a cut-off value of 7. There was significant survival difference (P<0.001) between the 2 groups. Figure 3. Distributions of SOFA score on POD 7 for the survivor group (n=72; A) and the non-survivor group (n=30; B). The case numbers decreased as the SOFA score increased in the survivor group but was dispersed in the non-survivor group.
Figure 3. Distributions of SOFA score on POD 7 for the survivor group (n=72; A) and the non-survivor group (n=30; B). The case numbers decreased as the SOFA score increased in the survivor group but was dispersed in the non-survivor group. Figure 4. Kaplan-Meier plot of post-transplant 3-month survival according to the number of independent risks. The more risks the patients carried, the higher mortality rates they would have.
Figure 4. Kaplan-Meier plot of post-transplant 3-month survival according to the number of independent risks. The more risks the patients carried, the higher mortality rates they would have. Tables
 Table 1. Demographic characteristics of 102 patients with post-transplant day 7 SOFA score >7.
Table 1. Demographic characteristics of 102 patients with post-transplant day 7 SOFA score >7. Table 2. Baseline demographics and clinical characteristics of high SOFA score patients by 3-month mortality.
Table 2. Baseline demographics and clinical characteristics of high SOFA score patients by 3-month mortality. Table 3. Uni-/multivariate analyses of clinical characteristics on the 3-month mortality in high POD 7 SOFA score patients by logistic regression.
Table 3. Uni-/multivariate analyses of clinical characteristics on the 3-month mortality in high POD 7 SOFA score patients by logistic regression. Table 4. Causes of death among non-survivors according to numbers of independent risks contained.
Table 4. Causes of death among non-survivors according to numbers of independent risks contained. Table 1. Demographic characteristics of 102 patients with post-transplant day 7 SOFA score >7.
Table 1. Demographic characteristics of 102 patients with post-transplant day 7 SOFA score >7. Table 2. Baseline demographics and clinical characteristics of high SOFA score patients by 3-month mortality.
Table 2. Baseline demographics and clinical characteristics of high SOFA score patients by 3-month mortality. Table 3. Uni-/multivariate analyses of clinical characteristics on the 3-month mortality in high POD 7 SOFA score patients by logistic regression.
Table 3. Uni-/multivariate analyses of clinical characteristics on the 3-month mortality in high POD 7 SOFA score patients by logistic regression. Table 4. Causes of death among non-survivors according to numbers of independent risks contained.
Table 4. Causes of death among non-survivors according to numbers of independent risks contained. Supplementary Table 1. The sequential organ failure assessment (SOFA) score and its components.
Supplementary Table 1. The sequential organ failure assessment (SOFA) score and its components. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








