04 May 2021: Review Paper
Current Status of Ultrasound in Acute Rejection After Renal Transplantation: A Review with a Focus on Contrast-Enhanced Ultrasound
Qiang Zhou1AEF, Yanjie Yu1AE, Wenhan Qin1BC, Youmin Pu1BE, Shuang Hu1D, Maozhi Tang1F, Xiaosong Xu1B, Hongwen Zhao1AE*DOI: 10.12659/AOT.929729
Ann Transplant 2021; 26:e929729
Abstract
ABSTRACT: Renal transplantation has developed into the best treatment for end-stage renal disease, but severe cases can even lead to loss of renal allograft function due to rejection and complications caused by surgical procedures. If a series of postoperative complications can be reduced or even avoided, the quality of life of recipients will be significantly improved. Acute rejection in a transplanted kidney is one of the main complications after renal transplantation. Early detection and diagnosis will significantly help the prognosis of transplanted kidney patients. As a seminal morphological and hemodynamic examination method, ultrasound can monitor the tissue structure and arteriovenous blood flow of the transplanted kidney, providing information on the transplanted kidney’s gross shape and blood perfusion. Ultrasound is a commonly used detection method after renal transplantation. At present, two-dimensional ultrasound, color Doppler ultrasound, three-dimensional ultrasound, and contrast-enhanced ultrasound have been applied in the monitoring of complications after renal transplantation. Contrast-enhanced ultrasound, as a non-invasive, radiation-free, and easy to perform examination technique, can qualitatively and quantitatively evaluate the microcirculatory blood perfusion of the transplanted kidney. It can reflect the function of the transplanted kidney more objectively and sensitively. In recent years, contrast-enhanced ultrasound has attracted attention as a new technology that can quantitatively monitor the transplanted kidney’s microcirculation perfusion. A large number of studies have shown that contrast-enhanced ultrasound has unique advantages in monitoring acute rejection after renal transplantation compared with other imaging methods, providing a reliable basis for clinical intervention. This article reviews the current status of and recent research on contrast-enhanced ultrasound in acute rejection after renal transplantation.
Keywords: Nephrology, Nephrology Nursing, Nephrostomy, Percutaneous, Graft Rejection, Kidney, Kidney Transplantation, Microcirculation, Quality of Life, Ultrasonography
Background
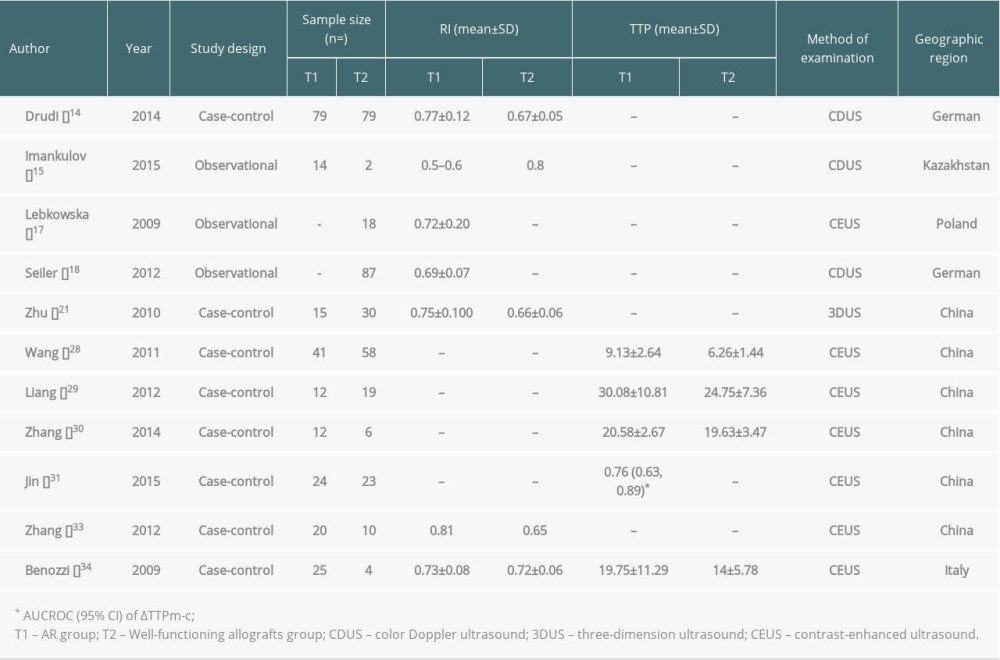
Allogeneic transplantation is an effective means to treat end-stage renal disease. Clinical practice has proved that complications after transplantation, especially the diagnosis and treatment of acute rejection (AR), have an important impact on patients’ survival and transplanted kidneys [1]. With the application of powerful immunosuppressive agents, the typical clinical manifestations of acute rejection are rare. The manifestations are mild, such as increased blood pressure and decreased urine output or renal function, making clinical diagnosis difficult [2]. Delayed graft function (DGF) is mainly caused by AR and acute tubular necrosis, and the clinical manifestations of AR and acute tubular necrosis are similar [3]. Hence, how to accurately and quickly differentiate between AR and acute tubular necrosis has also become an important topic in clinical research. Ultrasound is a non-invasive examination that can provide information on the morphology and blood perfusion of the transplanted kidney and indirectly reflects the transplanted kidney’s status, so it has become the most commonly used monitoring method after kidney transplantation [4]. B-mode ultrasound and color Doppler ultrasound are still routine diagnostic methods and are the main monitoring methods for kidney transplant recipients [5]. Color Doppler ultrasound is the most commonly used method for determining resistance index (RI). Although RI helps to detect acute renal insufficiency, it is still unable to differentiate the causes of renal insufficiency, including AR, chronic allograft dysfunction (CAD), and graft renal artery stenosis (TRAS) [6]. Contrast-enhanced ultrasound (CEUS) technology has been widely used in clinical practice [7]. The present article mainly reviews the current status of and recent research on CEUS in acute rejection after renal transplantation. Table 1 presents the results from our review of studies.
Pathological Classification and Manifestations of AR After Renal Transplantation
According to the
B-mode Ultrasound
B-mode ultrasound has a specific value in discovering the rejection reaction by observing the transplanted kidney’s morphology. It can monitor the morphology, size (volume, aggregate system size, and cortical thickness), internal structure, and perinephric condition of the transplanted kidney. When AR occurs after renal transplantation, the kidney appears full, the volume is enlarged, the renal parenchymal wall is thick, the echo is reduced, and the kidney contour is not smooth; in severe cases, the renal parenchymal echo is not uniformly distributed, and the renal pyramid is enlarged with edema [10]. B-mode ultrasound can detect borderline lesions and subclinical acute rejection through parenchymal swelling [11]. When AR after renal transplantation occurs, there are no obvious clinical symptoms or changes in the hemodynamic transplant index, indicating that the renal pyramidal veins are swollen, which reached a sensitivity of 96.2% [12]. However, B-mode ultrasound has difficulty distinguishing AR from acute tubular necrosis and other complications, lacking diagnostic specificity [13]. Therefore, more sensitive indicators need to be discovered.
Color Doppler Ultrasound
Color Doppler ultrasound can observe the blood perfusion in the kidney. When acute rejection occurs, the blood flow distribution in the kidney is asymmetrical, the arcuate arteries have almost no blood flow signals, and the interlobular arteries show intermittent flashing [14]. In terms of pulsed Doppler, the venous blood flow speed significantly increases during the AR period after renal transplantation for venous blood perfusion, resulting in a higher peak velocity during the diastolic period and an “arterial-like” pulsation phenomenon. The spectrum morphology of artery blood flow is abnormal during the AR period after renal transplantation for artery blood perfusion [15]. The systolic frequency spectrum rises sharply, showing a high sharp wave shape; the diastolic phase drops rapidly, the frequency harmonic is low and flat, and the blood flow is interrupted or disappears in some cases [15]. The above manifestations have a specific significance in the diagnosis of AR after kidney transplantation. The resistance index (RI) value has been an important research topic, and its utility in the ultrasound diagnosis of kidney transplantation is still controversial. In early research [16], it was believed that the increase in RI value had diagnostic significance for AR, but later studies found that the RI value was a non-specific indicator and was affected by a series of factors in the long-term prognosis of kidney transplant patients [17,18]. It can be considered that the utility of RI is limited in the diagnosis and differential diagnosis of AR and the differential diagnosis of different types of rejection.
Three-dimensional Ultrasound
The calculated volume of three-dimensional ultrasound is not affected by the geometry and position of the organ. It can measure the organ’s size more accurately, which makes it a good supplement to two-dimensional ultrasound. The combination of three-dimensional color power angiography (3D-CPA) and three-dimensional vascular volume imaging can evaluate kidney transplantation’s blood perfusion [19]. Wei et al [20] found that the three-dimensional reconstruction of the kidney volume in patients with AR was significantly larger than that in the normal group, and the results were similar to the comparison of the kidney volume before and after AR, suggesting that rejection is related to the increase in kidney volume. Zhu et al [21] reported that the sensitivity and specificity of vascularization flow index (VFI) in diagnosing AR after renal transplantation are 70.0% and 93.3%, vascularization index (VI) has 73.3% and 80.0%, flow index (FI) has 83.3% and 60.0%, mean gray value (MG) has 76.7% and 66.7%, RI has 60.0% and 73.3%, and pulsatility index (PI) has 66.7% and 53.3%, respectively. With VFI ≤18.78% as the critical value, prediction of AR has a positive predictive value of 60.8% and a negative predictive value of 93.4%. However, the parameter values of different pathological types of acute rejection have not been obtained yet.
Contrast-enhanced Ultrasound (CEUS)
CEUS can detect the blood flow of microcirculation perfusion of tissues and organs, improving the effectiveness of ultrasound diagnosis and may be a significant predictor of early AR after renal transplantation [22]. At present, the most widely used contrast agent is SonoVue (Bracco, Milan, Italy) [23]. SonoVue microbubbles do not pass through the capillary wall, and overflow into the interstitial space. Its hemodynamic changes are similar to red blood cells, with good stability. As a blood pool imaging agent, SonoVue microbubbles can reflect tissue microcirculation blood perfusion changes in real time. Unlike CT and MRI contrast agents, SonoVue microbubbles have no liver and kidney toxicity and are not metabolized by the kidneys, being excreted through breathing [24]. According to reports, the incidence of life-threatening adverse reactions in use of SonoVue microbubbles is only 0.001% [25]. The adverse reactions include headache and discomfort at the injection site, such as burning sensation, congestion, and paresthesia. SonoVue is mainly contraindicated in patients with right-to-left shunts, patients with severe pulmonary hypertension (pulmonary artery pressure >90 mmHg), uncontrolled systemic hypertension, adult respiratory distress syndrome, and cardiovascular disease. However, the safety and effectiveness of SonoVue have not been established in pregnant and lactating women, children, and adolescents [24]. As a rich blood supply organ, the kidney accounts for about 1/4 of the systemic blood flow, and the cortex and medulla of the kidney account for about 95% of the total kidney blood flow. Therefore, in most studies, the renal cortex and medulla are regarded as regions of interest [26]. After acquiring the contrast image for about 2 minutes, the image analysis software is used to analyze the dynamic image generated by the contrast image and to generate the time-intensity curve (TIC) of the region of interest in the cortex and medulla of the kidney. Multiple quantitative indicators, such as rise time (RT), time to peak (TTP), mean transit time (MTT), peak intensity (PI), and area under the ROC curve (AUC) were obtained [27]. Comparing the differences in these indicators between different groups can assist in the differential diagnosis of kidney transplant complications.
Wang et al [28] established that the contrast agent entered the kidney through the iliac artery. It presented “pulsatile” perfusion consistent with the heartbeat when AR after renal transplantation occurred. The distribution of the contrast agent was uneven, showing a stagnant area of contrast agent. The filling of SonoVue microbubbles in the renal cortex and medulla in the AR group was sparser than that of the normal renal function group, and the kidney contour showed a slightly “burr-like change”. The envelope of the TIC curve was rough, with apparent upward and downward fluctuations. The TIC curve slowly rose and fell, the peak of the curve became dull, and the second peak of the curve became lower. The curve’s span increased and even became flat and disappeared, showing a “single-peak” change. In contrast-enhanced ultrasound parameters, the perfusion time and emptying time were longer than those in the normal renal function group, and the RT and TTP of the cortex and medulla were also longer. Liang et al [29] analyzed the time-intensity curve of contrast-enhanced ultrasound in patients with acute renal transplant rejection and found that the absolute time to peak (ATTP) of the AR group was prolonged, and the rate of contrast agent velocity increased slowed. Compared with the normal renal function group, the contrast agent’s distribution was uneven in the AR group. The peak of the time-intensity curve was rounded, and the slope of the ascending branch increased, which has specific utility for the early diagnosis of AR after renal transplantation.
Zhang et al [30] analyzed the TIC parameters of 6 patients in the normal renal function group, 12 patients in the AR group, and 13 patients in the chronic rejection (CR) group after kidney transplantation. The experimental results showed that the AUC of the AR group and the CR group was lower than that in the normal renal function group (
Jin et al [31] performed a CEUS examination on 57 patients with kidney transplantation, including 24 patients in the normal renal function group, 23 patients with AR, and 10 patients with ATN confirmed by pathological biopsy. CEUS results showed that some of the AR group TIC time parameters were significantly longer than those in the normal renal function and ATN groups. The ROC curve was conducted to determine ΔRTm-c (the difference in RT between renal medulla and cortex) and ΔTTPm-c (the difference in TTP between the renal medulla and cortex), achieving the highest accuracy of the diagnosis of AR. The AUC values were 0.756 and 0.756, respectively. AR after kidney transplantation can cause diffuse inflammation and fibrinoid necrosis of small blood vessels in various parts of the transplanted kidney, stenosis or occlusion of the vessel lumen, and increase the perfusion resistance of the transplanted renal artery [32], which can decrease the microcirculation perfusion of the transplanted kidney and extend the perfusion time. Zhang et al [33] found that CEUS can dynamically detect the changes of microcirculation perfusion when AR after kidney transplantation occurs. The AUC of the AR after kidney transplantation group was significantly different from the normal renal function group. Benoni et al [34] conducted conventional ultrasound and contrast-enhanced ultrasound examinations on 39 patients after kidney transplantation, and the results showed that when the ratio-regional blood volume in the contrast-enhanced ultrasound parameters was less than 0.81, the ratio-mean transit time was less than 0.87, and the TTP was less than 18.5, the positive predictive value and negative predictive value were both significant.
In addition to ordinary angiography, intercellular adhesion molecule-1 (ICAM-1) and T cells (mostly targeted ultrasound contrast-enhanced T cells) are becoming another new type of imaging method for monitoring AR after kidney transplantation [35]. Normal renal tubular and vascular endothelial cells express only a small amount of ICAM-1 and no T cell infiltration. When AR occurs, the expression of ICAM-1 in the kidney increases significantly, and there is a large amount of T cell infiltration. A specific anti-ICAM-1 or T cell antibody is attached to the surface of the contrast agent. If AR occurs after kidney transplantation, the contrast agent can specifically bind to ICAM-1 or T cells for targeted positioning [36,37]. Grabner et al [23] investigated the suitability and feasibility of CEUS by using microbubbles targeted to CD3(+), CD4(+), and CD8(+) T cells in different rat models of renal disease, and the rats were divided into an AR group, an ATN group, a drug toxicity reaction group, and a contralateral orthotopic kidney group as a control group without transplantation. All the rats underwent targeted CEUS examination. The experimental results showed that T cell infiltration can be detected as soon as 2 days after transplantation. The AR group’s renal contrast signal was significantly stronger than that of the ATN group, drug toxicity reaction group, and contralateral orthotopic kidney group. The intensity of the contrast signal can also reflect the degree of AR. The higher the degree of AR, the stronger the contrast signal, which is confirmed by histopathology and immunohistochemistry. However, this experiment also had certain limitations. T cell infiltration cannot be observed in AR. For example, the ureteral infection can also have T cell infiltration, and targeted ultrasound contrast experiments are currently only used in animal experiments [23]. The specificity and safety of AR in routine clinical monitoring are not yet established.
Kidney transplantation is the terminal treatment for renal failure, and its functional status is crucial to the patient. Among the complications after kidney transplantation, acute rejection is still one of the main reasons for the poor short-term survival rate of patients [38]. Traditional two-dimensional ultrasound can observe the general morphological characteristics of transplantation, and color Doppler ultrasound can monitor the transplanted kidney’s intrarenal blood perfusion and measure blood flow parameters [39]. However, these 2 methods lack specificity and have limited value in the diagnosis of AR. Three-dimensional ultrasound measurements of the transplanted volume are more accurate than two-dimensional ultrasound and can obtain relevant blood vessel volume parameters, which provide a semi-quantitative index [20]. CEUS is highly sensitive to blood perfusion. By observing images and comparing various parameter values though CEUS, the occurrence of AR can be found sooner. It has the advantages of being real-time and non-invasive, strong reproducibility, and non-nephrotoxicity of the contrast agent, which have gradually led to increased clinical application [40]. However, recent research on CEUS has mostly consisted of small-sample studies, case reports, or animal studies of kidney transplantation. In addition, TIC parameters and the standards of normal and abnormal values need to be further improved by expanding the sample size. Since the evaluation parameters are still in the preliminary stage in the differential diagnosis of complications, as a diagnostic tool, CEUS lacks sufficient specificity, and currently cannot replace the criterion standards for detection of AR after kidney transplantation, such as renal biopsy and CTA examination. With the advancement of instruments and the improvement of clinical diagnostic techniques, it is believed that CEUS can become the main diagnostic method for AR after kidney transplantation in the future.
References
1. Zhang Q, Yu Z, Xu Y, Use of contrast-enhanced ultrasonography to evaluate chronic allograft nephropathy in rats and correlations between time-intensity curve parameters and allograft fibrosis: Ultrasound Med Biol, 2016; 42(7); 1574-83
2. Alvarez Rodriguez S, Hevia Palacios V, Sanz Mayayo E, The usefulness of contrast-enhanced ultrasound in the assessment of early kidney transplant function and complications: Diagnostics (Basel), 2017; 7(3); 53
3. Naesens M, Heylen L, Lerut E, Intrarenal resistive index after renal transplantation: N Engl J Med, 2013; 369(19); 1797-806
4. Korda D, Deak PA, Kozma V, Role of contrast-enhanced ultrasound in the follow-up of kidney transplant patients: Transplant Proc, 2016; 48(7); 2544-47
5. Granata A, Clementi S, Londrino F, Renal transplant vascular complications: The role of Doppler ultrasound: J Ultrasound, 2015; 18(2); 101-7
6. Cano H, Castaneda DA, Patino N, Resistance index measured by Doppler ultrasound as a predictor of graft function after kidney transplantation: Transplant Proc, 2014; 46(9); 2972-74
7. Zeisbrich M, Kihm LP, Druschler F, When is contrast-enhanced sonography preferable over conventional ultrasound combined with Doppler imaging in renal transplantation?: Clin Kidney J, 2015; 8(5); 606-14
8. Solez K, Colvin RB, Racusen LC, Banff 07 classification of renal allograft pathology: updates and future directions: Am J Transplant, 2008; 8(4); 753-60
9. Gillard R, Milicevic M, Follow-up of kidney transplant by medical imaging techniques: Rev Med Liege, 2019; 74(9); 484-87
10. Preuss S, Rother C, Renders L, Sonography of the renal allograft: Correlation between doppler sonographic resistance index (RI) and histopathology: Clin Hemorheol Microcirc, 2018; 70(4); 413-22
11. Krejci K, Zadrazil J, Tichy T, Sonographic findings in borderline changes and subclinical acute renal allograft rejection: Eur J Radiol, 2009; 71(2); 288-95
12. Gao BS, Wang YT, Wang G, A more sensitive hallmark of acute rejection (AR) after renal transplantation: Color doppler ultrasonography of renal pyramids: Human Immunology, 2011; 72(Suppl 1); S96
13. Sutherland T, Temple F, Chang S, Sonographic evaluation of renal transplant complications: J Med Imaging Radiat Oncol, 2010; 54(3); 211-18
14. Drudi FM, Liberatore M, Cantisani V, Role of color Doppler ultrasound in the evaluation of renal transplantation from living donors: J Ultrasound, 2014; 17(3); 207-13
15. Imankulov S, Doskali M, Oskenbaeva K, Evaluation of kidney allograft in the early posttransplant period using ultrasonography: Exp Clin Transplant, 2015; 13(Suppl 3); 62-65
16. Wang HK, Chou YH, Yang AH, Evaluation of cortical perfusion in renal transplants: Application of quantified power Doppler ultrasonography: Transplant Proc, 2008; 40(7); 2330-32
17. Lebkowska U, Janica J, Lebkowski W, Renal parenchyma perfusion spectrum and resistive index (RI) in ultrasound examinations with contrast medium in the early period after kidney transplantation: Transplant Proc, 2009; 41(8); 3024-27
18. Seiler S, Colbus SM, Lucisano G, Ultrasound renal resistive index is not an organ-specific predictor of allograft outcome: Nephrol Dial Transplant, 2012; 27(8); 3315-20
19. Schwarz C, Mühlbacher J, Böhmig GA, Impact of ultrasound examination shortly after kidney transplantation: Eur Surg, 2017; 49(3); 140-44
20. Wei W, Ai H, Wang J, Three-dimensional color Doppler ultrasonography in evaluation of acute renal transplant rejection: Chin J Med Imaging Techno, 2009; 25(1); 110-13 [in Chinese]
21. Zhu J, Xu M, Jiang Y, Value of three-dimensional ultrasonic imaging of vascular volume in assessing acute rejection of transplanted kidneys: Chin J Ultrasonogr, 2010(7); 583-85 [in Chinese]
22. Stenberg B, Wilkinson M, Elliott S, The prevalence and significance of renal perfusion defects in early kidney transplants quantified using 3D contrast enhanced ultrasound (CEUS): Eur Radiol, 2017; 27(11); 4525-31
23. Grabner A, Kentrup D, Pawelski H, Renal contrast-enhanced sonography findings in a model of acute cellular allograft rejection: Am J Transplant, 2016; 16(5); 1612-19
24. Kaspar M, Partovi S, Aschwanden M, Assessment of microcirculation by contrast-enhanced ultrasound: A new approach in vascular medicine: Swiss Med Wkly, 2015; 145; 14047
25. Dietrich CF, Greis CHow to perform contrast enhanced ultrasound: Dtsch Med Wochenschr, 2016; 141(14); 1019-24 [in German]
26. Grzelak P, Szymczyk K, Strzelczyk J, Perfusion of kidney graft pyramids and cortex in contrast-enhanced ultrasonography in the determination of the cause of delayed graft function: Ann Transplant, 2011; 16(1); 48-53
27. Greenbarg EH, Jimenez DA, Nell LA, Pilot study: Use of contrast-enhanced ultrasonography in feline renal transplant recipients: J Feline Med Surg, 2018; 20(4); 393-98
28. Wang J, Zhu J, Jiang G, Application of contrast-enhanced ultrasound in transplanted kidney with acute rejection: Chin J Med Ultrasound (Electronic Edition), 2011; 8(5); 1008-14 [in Chinese]
29. Liang W, Jiang L, Cai R, Contrast-enhanced ultrasonography time-intensity curve characteristics in transplantation kidney allograft acute pyelonephritis: Chin J Interv Imaging Ther, 2012; 9(6); 442-46 [in Chinese]
30. Zhang H, Liang W, Yu J, Quantitative analysis of contrast-enhanced ultrasonography in diagnosis of kidney transplantation rejection: Chin J Med Imaging, 2014; 22(9); 678-680 [in Chinese]
31. Jin Y, Yang C, Wu S, A novel simple noninvasive index to predict renal transplant acute rejection by contrast-enhanced ultrasonography: Transplantation, 2015; 99(3); 636-41
32. Shimizu T, Ishida H, Hayakawa N, Clinical and pathological analyses of cases of acute vascular rejection after kidney transplantation: Transplant Proc, 2017; 49(10); 2251-55
33. Zhang H, Li L, Li M, The diagnostic value of contrast-enhanced ultrasound in renal allograft rejection: J Med Imaging, 2012; 22(3); 479-81 [in Chinese]
34. Benozzi L, Cappelli G, Granito M, Contrast-enhanced sonography in early kidney graft dysfunction: Transplant Proc, 2009; 41(4); 1214-15
35. Wu W, Zhang Z, Zhuo L, Ultrasound molecular imaging of acute cellular cardiac allograft rejection in rat with T-cell-specific nanobubbles: Transplantation, 2013; 96(6); 543-49
36. Grabner A, Kentrup D, Muhlmeister M, Noninvasive imaging of acute renal allograft rejection by ultrasound detection of microbubbles targeted to T-lymphocytes in rats: Ultraschall Med, 2016; 37(1); 82-91
37. Fischer K, Ohori S, Meral FC, Testing the efficacy of contrast-enhanced ultrasound in detecting transplant rejection using a murine model of heart transplantation: Am J Transplant, 2017; 17(7); 1791-801
38. Augustine J, Kidney transplant: New opportunities and challenges: Cleve Clin J Med, 2018; 85(2); 138-44
39. Granata A, Di Nicolò P, Scarfia VR, Renal transplantation parenchymal complications: what Doppler ultrasound can and cannot do: J Ultrasound, 2015; 18(2); 109-16
40. Hai Y, Chong W, Liu JB, The diagnostic value of contrast-enhanced ultrasound for monitoring complications after kidney transplantation – a systematic review and meta-analysis: Acad Radiol, 2020 [Online ahead of print]
In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860