09 February 2021: Original Paper
Association Between Trough Level of Tacrolimus and Change in Estimated Glomerular Filtration Rate 1 Year After Living Donor Liver Transplantation
Kumiko Muta1ABCDEF*, Mineaki Kitamura1ABCDE, Masaaki Hidaka2BE, Yuki Ota1AB, Takanobu Hara2BE, Akihiko Soyama2BD, Satoshi Miuma3BE, Hisamitsu Miyaaki3BE, Kazuhiko Nakao3BE, Susumu Eguchi2BDE, Hiroshi Mukae4DE, Tomoya Nishino1ADEDOI: 10.12659/AOT.928858
Ann Transplant 2021; 26:e928858
Abstract
BACKGROUND: Although the risk factors for chronic kidney disease progression after deceased donor liver transplantation have been widely reported, there are few reports describing the factors associated with kidney function changes in patients after living donor liver transplantation (LDLT). This study aims to further investigate these kidney function change factors.
MATERIAL AND METHODS: This retrospective study was performed using the data of patients who underwent LDLT at the Nagasaki University Hospital, Japan from August 2000 to November 2017. Factors contributing to post-transplantation estimated glomerular filtration rate (eGFR) changes were analyzed.
RESULTS: A total of 191 cases were reviewed. The average age was 53.8 years, and 108 (56.5%) patients were male. Compared to pre-transplantation eGFR levels, eGFR 1 year after LDLT improved in 65 patients (34%) and deteriorated in 126 patients (66%). Multivariate regression analysis revealed that pre-transplant diuretics (P=0.04) and tacrolimus trough value 1 year after transplantation (P=0.04) were significantly associated with elevated eGFR changes. eGFR elevation 1 year after LDLT was more pronounced in patients with a low tacrolimus trough level 1 year after LDLT (P=0.01). Therefore, mycophenolate mofetil was added to tacrolimus in patients with poor renal function before LDLT.
CONCLUSIONS: Tacrolimus trough level was associated with eGFR changes 1 year after LDLT. The adjusted dose of tacrolimus and combined use of other immunosuppressants may be important to maintain renal function after LDLT.
Keywords: Glomerular Filtration Rate, Liver Transplantation, Living Donors, Tacrolimus, End stage liver disease, Immunosuppressive Agents, Severity of Illness Index
Background
Liver transplantation outcomes, such as graft survival period, have improved because of advances in surgery and immunosuppressant usage. In fact, a previous study [1] found that 1-year survival following liver transplantation increased from 66% in 1987 to 92% in 2015. Accordingly, the number of liver transplant recipients with chronic complications, including chronic graft rejection, chronic kidney disease (CKD), and cardiovascular diseases, has increased [2]. Among them, severe CKD is associated with poor patient survival [3]. CKD after liver transplantation is multifactorial. A previous analysis revealed that the female sex, older age, pre-transplantation proteinuria, low glomerular filtration rate (GFR) 1 year after transplantation, and high creatinine 6 months after transplantation were associated with CKD progression in patients who received deceased donor liver transplants [3]. Another study revealed that pre-transplant diabetes mellitus, urinary tract infections, and hypercholesterolemia within 1 year of undergoing deceased donor liver transplantation were CKD stage progression risk factors [4].
In European countries and the United States, the proportion of patients undergoing deceased donor liver transplantation is higher than that of patients undergoing living donor liver transplantation (LDLT) [5,6]. In contrast, the number of LDLTs is extremely high in Japan. For example, of the total number of liver transplantation cases in Japan in 2017, 83.4% were of LDLT [7]. The recognition and management of risk factors for acute kidney injury and CKD after LDLT have been suggested to improve the post-transplantation prognosis [8]. Although the causes for CKD progression and estimated GFR (eGFR) changes after deceased donor liver transplantation have been previously reported, there are few reports describing the factors affecting renal function after LDLT.
The association between calcineurin inhibitors (CNIs) and kidney dysfunction progression after liver transplantation is controversial. In particular, the relationship between tacrolimus (TAC) and kidney dysfunction after LDLT has not been clarified.
Although we elucidated the association between diuretics and kidney function in a previously reported retrospective study [9], the aim of the present study was to analyze the factors, specifically CNIs, contributing to changes in kidney function after LDLT.
Material and Methods
PATIENT POPULATION:
We retrospectively analyzed the data of all patients aged 18 years and older who underwent LDLT at the Nagasaki University Hospital, Japan between August 2000 and November 2017. Patients who were younger than 18 years at the time of LDLT and those from other countries were excluded because the modified Modification of Diet in Renal Disease (MDRD) Study equation for the Japanese population could not be applied to them [10]. In addition, patients who died within 1 year of receiving the transplant or those who were on maintenance hemodialysis, both before and after transplantation, were excluded. Among the eligible patients, recipients whose laboratory results could be examined were included.
Transplant recipient management and donor selection process have been indicated previously [11,12]. Graft selection was based on the estimated liver volume calculated using multidetector computed tomography. Rituximab (375 mg/m2) was used before LDLT in ABO-incompatible transplantations. Immunosuppression after LDLT was mainly managed with tacrolimus and a steroid. The trough level of tacrolimus was adjusted to 10–15 and 5–8 ng/mL within 1 month and at 1 month from LDLT, respectively. In contrast, the steroid was tapered within the first 3 months after transplantation. In cases with mild kidney dysfunction before and after LDLT, mycophenolate mofetil was additionally administered to the patients early after LDLT. Furthermore, the trough level of tacrolimus in such cases was adjusted to 5–8 and 3–5 ng/mL within 1 month and at 1 month after LDLT, respectively. The dose of mycophenolate mofetil was adjusted following platelet counts evaluation. In cases with deterioration of kidney function after LDLT, basiliximab and the steroid were first administered, while tacrolimus was then started with careful attention to avoid acute kidney injury. Finally, if symptoms associated with neurological adverse effects occurred in recipients, tacrolimus was switched to cyclosporine (ie, a calcineurin inhibitor).
METHODS:
Patient characteristics and preoperative and postoperative laboratory results were collected from medical records. All patients were followed up for more than 1 year after LDLT, and blood and urine test results, which were performed within 1 month preoperatively and about 1 year postoperatively, were used. The modified MDRD Study equation for the Japanese population was used to assess the kidney function as follows: eGFR=194×(serum creatinine)−1.094×(age)−0.287×0.739 (if female) [10]. The analyzed outcome was the %eGFR change over 1 year (from before LDLT to after LDLT), which was calculated as follows: %eGFR change over 1 year (%)=100×{(eGFR 1 year after LDLT–eGFR before LDLT)/eGFR before LDLT} [13]. Patients were divided into the improving and deteriorating groups based on the values of the %eGFR changes over 1 year, and the contributing factors for both groups were analyzed.
STATISTICAL ANALYSIS:
Statistical analyses were performed using JMP®PRO14 (SAS Institute Inc., Cary, NC, USA), with a
The Ethics Committee of Nagasaki University Hospital (Nagasaki, Japan) (19041518) approved this research, and all patients were informed about the details of this study in the opt-out and could refuse participation in the study if they desired to do so.
Results
BASELINE PATIENT CHARACTERISTICS:
Of the 242 patients who underwent LDLT at our hospital, 1 patient who was younger than 18 years at the time of transplantation, 48 patients who died within 1 year of receiving LDLT because of liver failure and infections, and 1 patient who was on maintenance hemodialysis both before and after LDLT were excluded. The laboratory results of 1 patient were not available for assessment after the first year after LDLT. Finally, the data of 191 recipients were studied. The baseline characteristics are shown in Table 1. The average patient age was 53.8 years, and 108 patients (56.5%) were male. The main etiologies for liver cirrhosis were HCV infection (36.1%), hepatitis B virus infection (17.8%), and alcoholic cirrhosis (14.7%). The average Model for End-Stage Liver Disease score was 18.0. The mean pre-transplant eGFR was 76.1 mL/min/1.73 m2. CNIs were administered to 169 patients (88.5%) after transplantation; specifically, TAC was administered to 153 patients (80.1%) and MMF to 114 patients (59.7%).
CHANGES IN EGFR VALUES:
The average eGFR decreased to 65.2 mL/min/1.73 m2 1 year after LDLT. The average change in the eGFR percentage over 1 year was −14.0% (−32.9 to 12.2). Of the 191 patients, 65 patients (34%) showed elevated %eGFR changes over 1 year (improving group) and the remaining 126 patients (66%) showed decreased %eGFR changes over 1 year (deteriorating group).
FACTORS CONTRIBUTING TO EGFR CHANGES OVER 1 YEAR:
Table 2 shows the contributory factors for eGFR changes over 1 year by comparing the %eGFR changes over 1 year between the improving and deteriorating groups. In the improving group, the diuretics prescription rate was higher than that in the deteriorating group (p=0.007). The pre-transplant hemoglobin, pre-transplant eGFR, and TAC trough values 1 year after LDLT were significantly lower in the improving group than in the deteriorating group (p=0.004, p<0.001, and p=0.008, respectively).
LOGISTIC REGRESSION ANALYSIS FOR ELEVATED EGFR 1 YEAR AFTER LDLT:
We analyzed the factors contributing to elevated eGFR levels 1 year after LDLT using univariate regression analysis (Table 3). The univariate regression analysis revealed that diuretics prescription before LDLT was associated with eGFR changes. In addition, a low pre-transplant hemoglobin, pre-transplant eGFR, trough values of TAC 1 year after LDLT, and trough value of TAC 1 year after LDLT <5.0 ng/mL were associated with elevated %eGFR changes over 1 year. A multivariate regression analysis of the factors with low P values in the univariate regression analysis revealed that only a low pre-transplant eGFR significantly contributed to the elevation of %eGFR change over 1 year (P<0.001) (Table 4). Since pre-transplant eGFR and the elevation of %eGFR changes over 1 year were strongly associated, another multivariate regression analysis was performed excluding the pre-transplant eGFR. Pre-transplant diuretics usage (P=0.04) and low TAC trough values 1 year after LDLT (P=0.04) were found to be significant contributory factors. Although patients who had been administered with cyclosporine within 1 year from LDLT were included in these analysis, the results of logistic regression analysis in patients who had not been administered with cyclosporine within 1 year from LDLT also exhibited the similar tendency with the above-mentioned (Supplementary Tables 1, 2).
RELATIONSHIP BETWEEN TAC TROUGH VALUES 1 YEAR AFTER LDLT AND EGFR CHANGES:
To evaluate the association between TAC trough values and eGFR changes, patients were divided into 4 groups based on TAC trough values 1 year after LDLT. The Wilcoxon rank test indicated that the group with the lowest trough value showed the highest eGFR improvement and that there were inverse relationships between TAC trough levels and the %eGFR changes (p=0.04) (Figure 1). Next, the trend between TAC trough values and %eGFR changes over 1 year was examined using the Cochran-Armitage test. In the improving group, we found that the lower the TAC trough values 1 year after LDLT, the higher the number of cases showing elevated eGFR levels (Figure 2). In contrast, the %eGFR changes over 1 year revealed the opposite trend in the deteriorating group.
In the association between immunosuppressant usage and kidney function, pre-transplant eGFR was significantly higher in patients who were only prescribed TAC (84.2±29.2 mL/min/1.73 m2) than in those who were prescribed a combination of TAC and MMF (72.6±27.6 mL/min/1.73 m2) at our hospital (
Discussion
In this study, the factors contributing to changes in kidney function were examined in patients who had undergone LDLT. The use of pre-transplant diuretics and TAC trough values 1 year after LDLT were significantly correlated with eGFR changes, except for pre-transplant eGFR. Particularly, eGFR elevation 1 year after LDLT was more pronounced in patients with low TAC trough values 1 year after LDLT. This suggests that the TAC trough values play an important role in determining eGFR value changes after LDLT.
Although there are few reports on the causes of CKD progression and eGFR changes after LDLT, previous reports have suggested that advanced age, low pre-transplant eGFR value, and graft-to-recipient weight ratio as negative predictors were the associated risk factors for CKD progression after LDLT [2,14].
TAC is mainly used for the prevention and treatment of graft rejection following various solid organ transplantations [15]. However, TAC causes vascular dysfunction resulting from an increase in vasoconstrictors such as endothelin and thromboxane and a decrease in vasodilators such as prostacyclin and prostaglandin E2 [16]. These changes may induce arteriolopathy, interstitial fibrosis, and tubular atrophy manifesting as chronic nephrotoxicity [16]. To avoid nephrotoxicity, frequent monitoring, dose adjustments, and reduced TAC doses in combination with MMF, azathioprine, or everolimus are considered [17,18]. With respect to liver transplantation, the association between TAC and kidney dysfunction is controversial. A previous study showed that the average plasma TAC concentration was an independent risk factor for new-onset CKD in patients after liver transplantation [19]. Another study revealed that TAC usage was associated with a low risk of developing CKD in patients who underwent deceased orthotopic liver transplantation [3]. In the current study, we observed that the TAC trough value had a great impact on eGFR changes, and eGFR showed a vast improvement if the TAC trough value was lower. The recommended TAC trough blood concentration is 4 to 6 ng/mL at more than 3 months after transplantation and 3 to 5 ng/mL at more than 12 months after transplantation in adult liver transplantation recipients according to a clinical guideline for transplant medications [15]. Following the abovementioned guideline, we speculate that the ideal value would be under 5 ng/mL because the average TAC trough value related to the %eGFR changes in the improving group was 5.2 ng/mL, and the TAC trough value of less than 5.0 ng/mL 1 year after LDLT was associated with elevation of %eGFR changes over 1 year in this study. As MMF was combined with TAC in patients with a poor renal function before LDLT in this retrospective survey, we believe that it is advisable to treat patients with a low eGFR using a reduced TAC concentration and/or MMF at the time of LDLT to maintain renal function and avoid graft rejection.
There were several limitations to this study. This was a retrospective study, and limited data were available. Moreover, the results of this study may only be applicable to Japanese patients. There were only 2 cases in which everolimus was administered in this study because everolimus was not covered by the Japanese health insurance system as an immunosuppressant after liver transplantation throughout the observation period. Although eGFR changes 1 year after LDLT with respect to the pre-transplant levels were analyzed, pre-transplant CKD was not taken into consideration. In fact, 36 patients were diagnosed with CKD based on their serum creatinine levels 3 months prior to the LDLT procedures in this study. Determining the complications, such as infection and rejection, is essential to decide whether patients could be maintained at a lower trough level of tacrolimus after LDLT. However, such factors were not considered in this study. Future studies assessing these factors are warranted. TAC trough values were assessed at only 1 point 1 year after LDLT, and the transition of TAC trough levels was not considered. Future studies are needed to further analyze these matters.
Conclusions
This study analyzed the factors contributing to kidney function changes after LDLT. As the TAC trough values were associated with eGFR changes after LDLT, the adjusted dose of TAC and combined use of other immunosuppressants may be important to maintain renal function after LDLT.
Figures
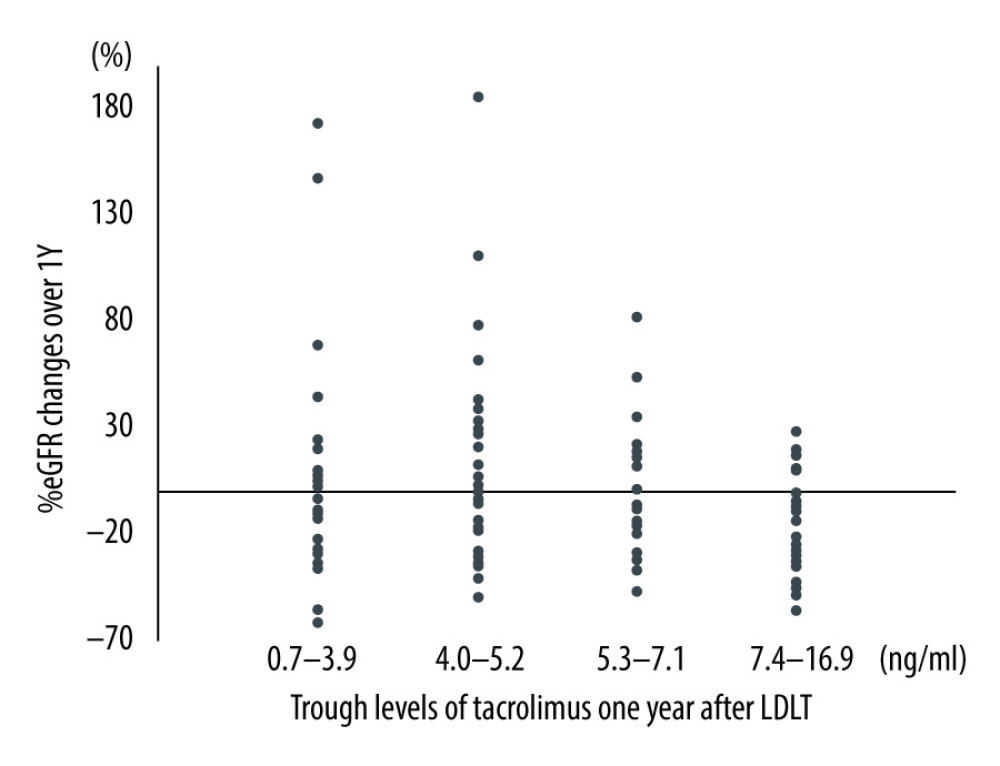 Figure 1. The relationship between trough value of tacrolimus 1 year after living donor liver transplantation and %eGFR changes over 1 year after living donor liver transplantation (p=0.04). One patient who was administered cyclosporine after LDLT and who had switched to tacrolimus within 1 year after the transplantation was included here.
Figure 1. The relationship between trough value of tacrolimus 1 year after living donor liver transplantation and %eGFR changes over 1 year after living donor liver transplantation (p=0.04). One patient who was administered cyclosporine after LDLT and who had switched to tacrolimus within 1 year after the transplantation was included here. 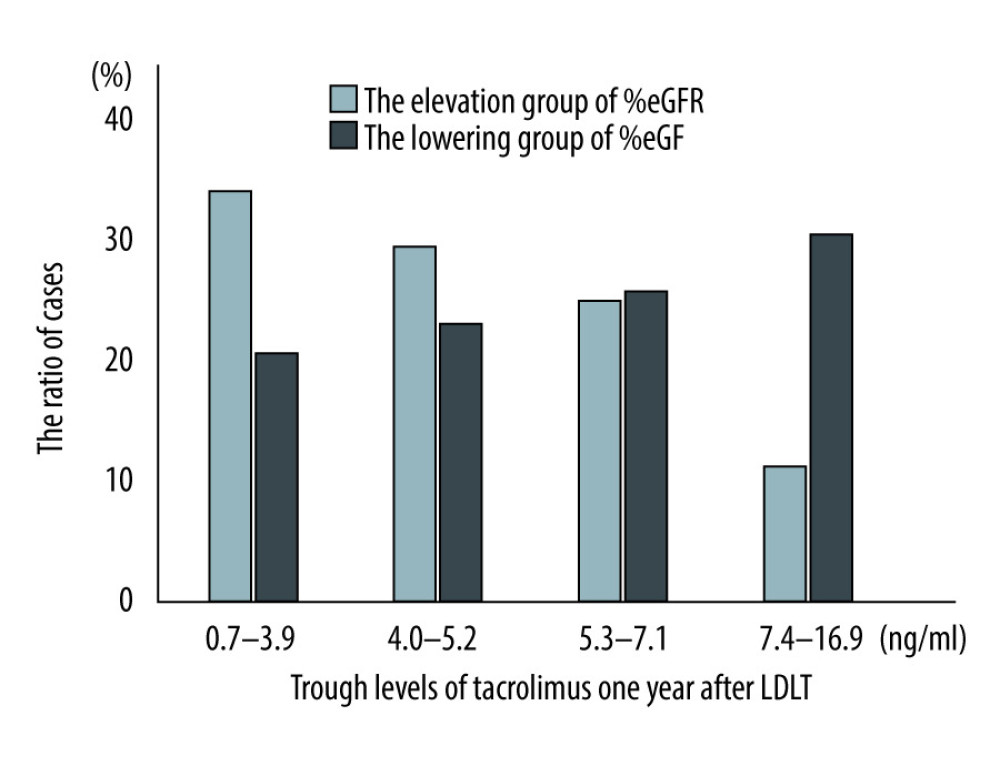 Figure 2. Cochran-Armitage test between trough values of tacrolimus and %eGFR changes over 1 year after living donor liver transplantation (ptrend=0.01). LDLT – living donor liver transplantation.
Figure 2. Cochran-Armitage test between trough values of tacrolimus and %eGFR changes over 1 year after living donor liver transplantation (ptrend=0.01). LDLT – living donor liver transplantation. Tables
Table 1. Patient baseline characteristics.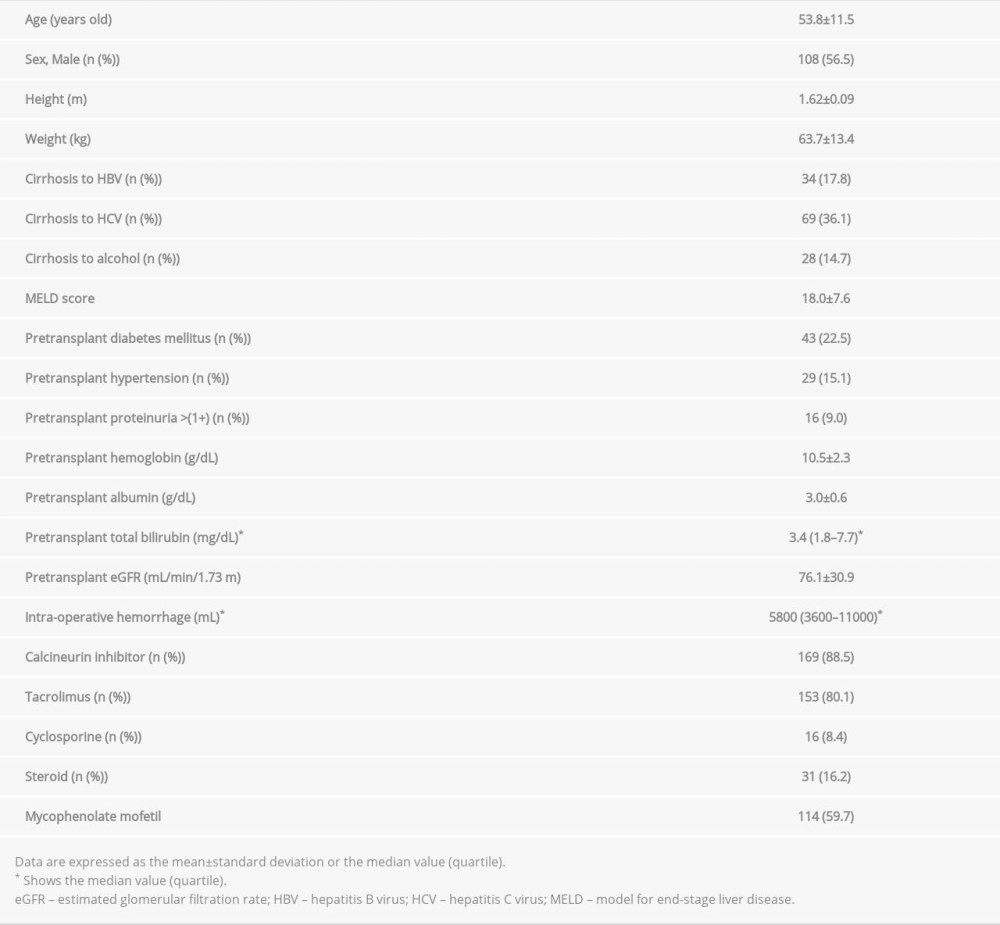 Table 2. The comparison between the ameliorating and deteriorating group of eGFR change over 1 year after living donor liver transplantation.
Table 2. The comparison between the ameliorating and deteriorating group of eGFR change over 1 year after living donor liver transplantation.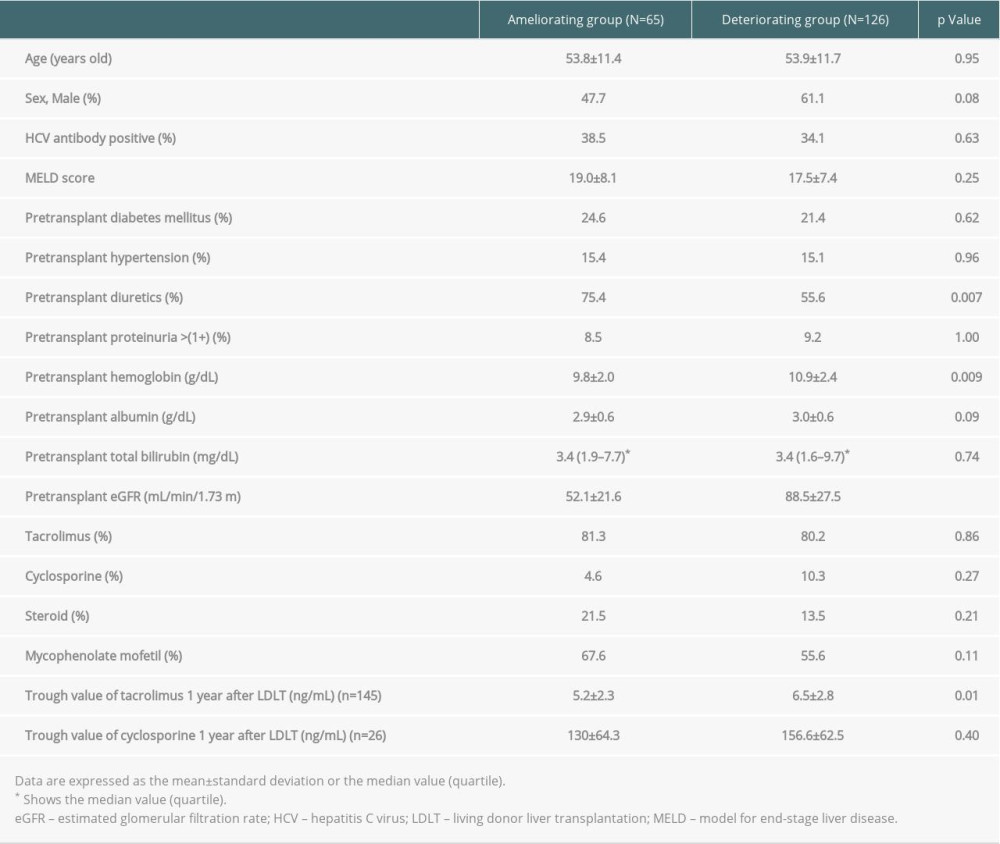 Table 3. Univariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation.
Table 3. Univariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation.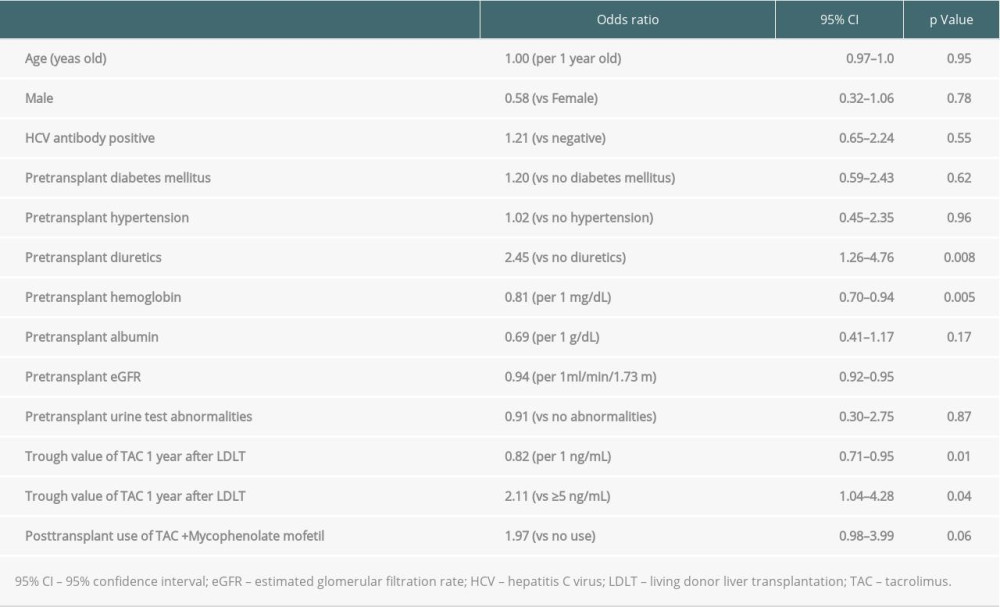 Table 4. Multivariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation.
Table 4. Multivariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation.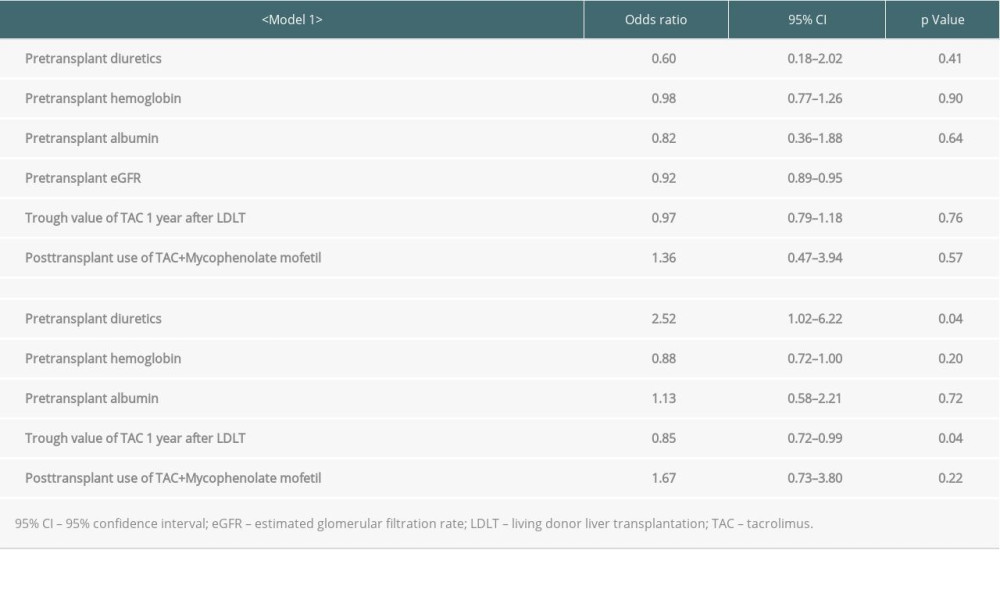 Supplementary Table 1. Univariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation in patients who were not administered with cyclosporine.
Supplementary Table 1. Univariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation in patients who were not administered with cyclosporine.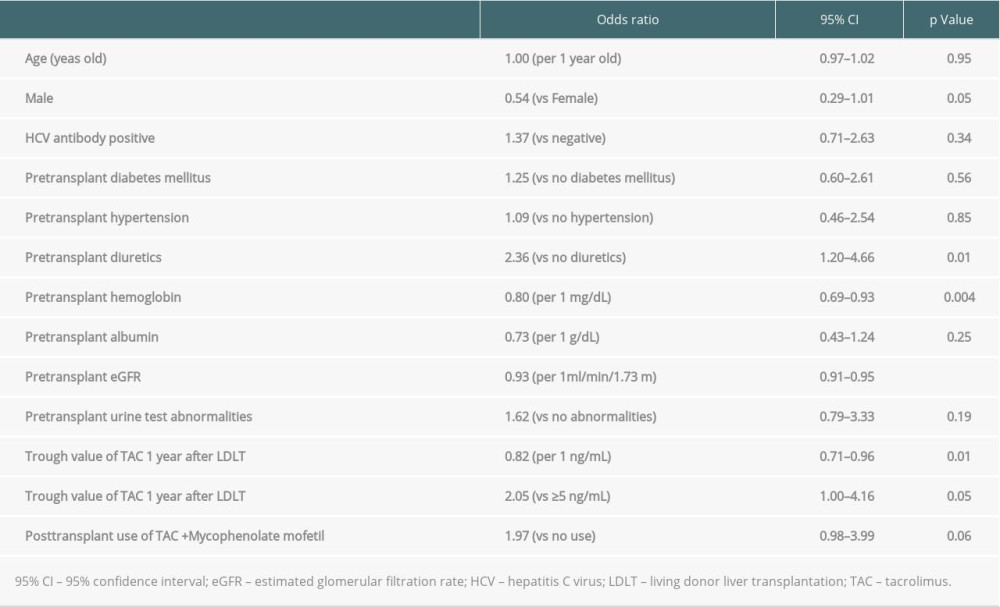 Supplementary Table 2. Multivariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation in patients who were not administered with cyclosporine.
Supplementary Table 2. Multivariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation in patients who were not administered with cyclosporine.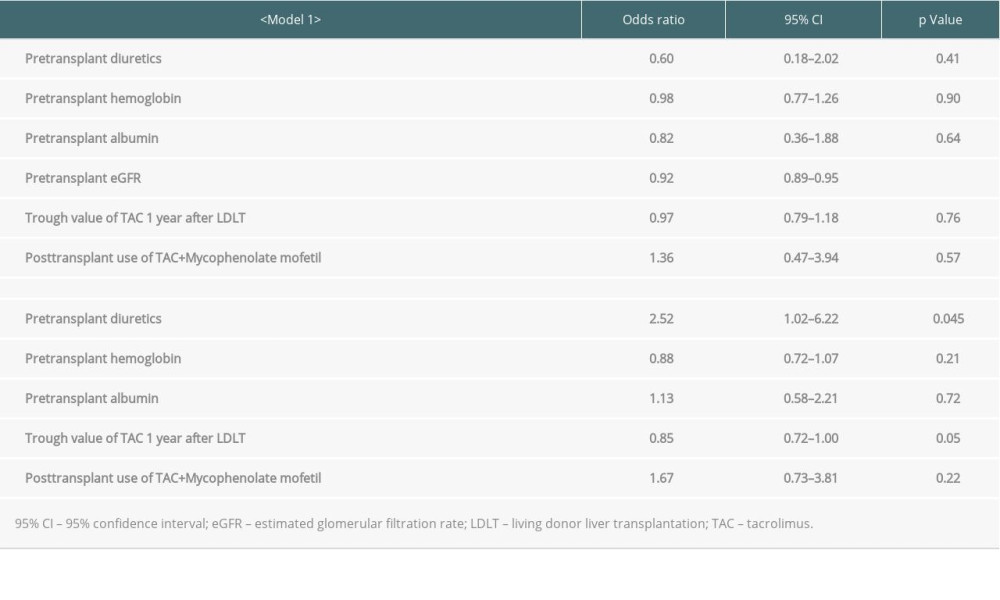
References
1. Rana A, Ackah RL, Webb GJ, No gains in long-term survival after liver transplantation over the past three decades: Ann Surg, 2019; 269; 20-27
2. Nishi H, Shibagaki Y, Kido R, Chronic renal outcome after living donor liver transplantation: Clin Transplant, 2013; 27; 90-97
3. O’Riordan A, Wong V, McCormick PA, Chronic kidney disease post-liver transplantation: Nephrol Dial Transplant, 2006; 21; 2630-36
4. Lamattina JC, Foley DP, Mezrich JD, Chronic kidney disease stage progression in liver transplant recipients: Clin J Am Soc Nephrol, 2011; 6; 1851-57
5. Kwong A, Kim WR, Lake JR, OPTN/SRTR 2018 Annual Data Report: Liver: Am J Transplant, 2020; 20(Suppl s1); 193-299
6. Shukla A, Vadeyar H, Rela M, Shah S, Liver transplantation. East versus West: J Clin Exp Hepatol, 2013; 3; 243-53
7. Liver transplantation in Japan – registry by the Japanese Liver Transplantation Society: Isyoku, 2017; 51; 145-59 [in Japanese]
8. Inoue Y, Soyama A, Takatsuki M, Does the development of chronic kidney disease and acute kidney injury affect the prognosis after living donor liver transplantation?: Clin Transplant, 2016; 30; 518-27
9. Kitamura M, Hidaka M, Muta K, Prediction of liver prognosis from pre-transplant renal function adjusted by diuretics and urinary abnormalities in adult-to-adult living donor liver transplantaion: Ann Transplant, 2020; 25; e924805
10. Imai E, Horio M, Nitta K, Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease: Clin Exp Nephrol, 2007; 11; 41-50
11. Hidaka M, Eguchi S, Takatsuki M, The Kupffer cell number affects the outcome of living donor liver transplantation from elderly donors: Transplant Direct, 2016; 2; e94
12. Eguchi S, Takatsuki M, Hidaka M, Evolution of living donor liver transplantation over 10 years: Experience of a single center: Surg Today, 2008; 38; 795-800
13. Coresh J, Turin TC, Matsushita K, Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality: JAMA, 2014; 311; 2518-31
14. Abdel-Khalek EE, Alrefaey AK, Yassen AM, Renal dysfunction after living-donor liver transplantation. Experience with 500 cases: J Transplant, 2018; 2018 5910372
15. TRANSPLANT BC, Tacrolimus. Immediate release and extended release: Clinical guidelines for transplant medications, 2019; Chapter 10; 75-85
16. Naesens M, Kuypers DR, Sarwal M, Calcineurin inhibitor nephrotoxicity: Clin J Am Soc Nephrol, 2009; 4; 481-508
17. Samuel D, Sanchez-Fueyo A, Immunotherapy in liver transplantation: J Hepatol, 2017; 67; 874-75
18. De Simone P, Carrai P, Coletti L, Everolimus vs mycophenolate mofetil in combination with tacrolimus: A propensity score-matched analysis in liver transplantation: Transplant Proc, 2018; 50; 3615-20
19. Li Y, Li B, Wang W, Lv J, Risk factors for new-onset chronic kidney disease in patients who have received a liver transplant: Exp Ther Med, 2018; 15; 3589-95
Figures
 Figure 1. The relationship between trough value of tacrolimus 1 year after living donor liver transplantation and %eGFR changes over 1 year after living donor liver transplantation (p=0.04). One patient who was administered cyclosporine after LDLT and who had switched to tacrolimus within 1 year after the transplantation was included here.
Figure 1. The relationship between trough value of tacrolimus 1 year after living donor liver transplantation and %eGFR changes over 1 year after living donor liver transplantation (p=0.04). One patient who was administered cyclosporine after LDLT and who had switched to tacrolimus within 1 year after the transplantation was included here. Figure 2. Cochran-Armitage test between trough values of tacrolimus and %eGFR changes over 1 year after living donor liver transplantation (ptrend=0.01). LDLT – living donor liver transplantation.
Figure 2. Cochran-Armitage test between trough values of tacrolimus and %eGFR changes over 1 year after living donor liver transplantation (ptrend=0.01). LDLT – living donor liver transplantation. Tables
 Table 1. Patient baseline characteristics.
Table 1. Patient baseline characteristics. Table 2. The comparison between the ameliorating and deteriorating group of eGFR change over 1 year after living donor liver transplantation.
Table 2. The comparison between the ameliorating and deteriorating group of eGFR change over 1 year after living donor liver transplantation. Table 3. Univariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation.
Table 3. Univariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation. Table 4. Multivariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation.
Table 4. Multivariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation. Table 1. Patient baseline characteristics.
Table 1. Patient baseline characteristics. Table 2. The comparison between the ameliorating and deteriorating group of eGFR change over 1 year after living donor liver transplantation.
Table 2. The comparison between the ameliorating and deteriorating group of eGFR change over 1 year after living donor liver transplantation. Table 3. Univariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation.
Table 3. Univariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation. Table 4. Multivariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation.
Table 4. Multivariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation. Supplementary Table 1. Univariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation in patients who were not administered with cyclosporine.
Supplementary Table 1. Univariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation in patients who were not administered with cyclosporine. Supplementary Table 2. Multivariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation in patients who were not administered with cyclosporine.
Supplementary Table 2. Multivariate logistic regression analysis for elevated eGFR 1 year after living donor liver transplantation in patients who were not administered with cyclosporine. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








