17 November 2020: Original Paper
Treatment of Antibody-Mediated Rejection After Kidney Transplantation: Immunological Effects, Clinical Response, and Histological Findings
Marcos Vinicius de Sousa1ABCDEF*, Ana Claudia Gonçalez2CD, Ricardo de Lima Zollner3DE, Marilda Mazzali1ADEDOI: 10.12659/AOT.925488
Ann Transplant 2020; 25:e925488
Abstract
BACKGROUND: Antibody-mediated rejection (AMR) presents with diverse clinical manifestations and can have a potential negative impact on graft function and survival. If not treated successfully, AMR can lead to 20-30% graft loss after 1 year. Little is known about the efficacy of AMR treatment, and the most appropriate therapeutic strategy has not yet been determined. This study evaluated the effects of AMR treatment with plasmapheresis (PP) and intravenous immunoglobulin (IVIG) on renal function, intensity of anti-HLA antibodies, and graft biopsy morphology.
MATERIAL AND METHODS: This single-center retrospective cohort study included renal transplant recipients with biopsy-proven AMR who were treated with PP and/or IVIG. Clinical findings, mean fluorescence intensity of donor-specific anti-HLA antibodies (DSA), and graft histology findings, classified according to Banff score at the time of AMR and 6 and 12 months later, were evaluated.
RESULTS: Of the 42 patients who met the inclusion criteria, 38 (90.5%) received IVIG and 26 (61.9%) underwent PP. At AMR diagnosis, 36 (85.7%) patients had proteinuria, with their estimated glomerular filtration rate remaining stable during follow-up. During the first year, 8 (19.0%) patients experienced graft failure, but none died with a functioning graft. Reductions in the class I panel of reactive antibodies were observed 6 and 12 months after AMR treatment, with significant reductions in DSA-A and -B fluorescence intensity, but no changes in DSA-DQ. Graft biopsy showed reductions in inflammation and C4d scores, without improvements in microvascular inflammation.
CONCLUSIONS: AMR treatment reduced biopsy-associated and serological markers of AMR, but did not affect DSA-DQ.
Keywords: Biopsy, Graft Rejection, Graft Survival, HLA Antigens, Immunoglobulins, Intravenous, Plasmapheresis, Glomerular Filtration Rate, Isoantibodies, Kidney Transplantation, Proteinuria
Background
About 6.7% of kidney transplant recipients experience antibody-mediated rejection (AMR) [1]. If not successfully treated, an estimated 20–30% of patients with AMR experience allograft loss within 1 year [2]. The main antigenic targets of AMR are the human leukocyte antigens (HLAs), molecules expressed at the surface of nucleated cells with allorecognition function [2]. Previous exposure to foreign HLAs, such as during pregnancy, blood transfusion, or transplantation, can elicit the production of anti-HLA antibodies, increasing the risk of AMR following kidney transplantation [1,2]. In addition to preformed donor-specific anti-HLA antibodies (DSA),
The presence of DSA is a crucial component for the diagnosis of AMR in kidney transplant recipients [3]. DSA can be detected by 2 methods: cell-based tests, including complement-dependent lymphocytotoxicity and flow cytometric crossmatch assays; and solid-phase tests, including enzyme-linked immunosorbent assays and multianalyte single-bead tests by flow cytometry or Luminex assays [2]. Furthermore, the diagnosis of AMR requires biopsy evidence of current or recent antibody-vascular endothelium interaction, with identification of tissue deposits of C4d, a digestion product of the complement component C4, and evidence of microvascular inflammation (MVI) and/or macrovascular lesions [3]. C4d deposits can be detected by immunoperoxidase and immunofluorescence assays [3], whereas graft MVI can be detected histologically by capillary dilatation, endothelial cell cytoplasmic swelling or enlargement, and vacuolization. Macrovascular lesions present with severe intimal arteritis and monocytic and lymphocytic inflammation of the intima, with or without transmural necrosis [3].
AMR is a disease process with a continuum of severity, varying from subclinical indolent microvascular abnormalities to chronic damage, dysfunction, and graft loss [3]. The aims of AMR treatment are the removal of harmful alloantibodies from the circulation, with plasmapheresis (PP) or immunoadsorption; and the modulation of components of acquired and innate immunity, by treatment with intravenous immunoglobulin (IVIG), the anti-CD20 antibody rituximab, the proteasome inhibitor bortezomib, the anti-C5 antibody eculizumab, or splenectomy [1,4]. PP promptly removes formed antibodies and is associated with an 80–90% reversal of AMR and 80% graft survival at 18 months [2], whereas IVIG is potentially useful due to its immunomodulatory effects [1]. The monoclonal anti-CD20 antibody rituximab binds to the surface of precursor and mature B cells, resulting in transient B cell depletion [1]. PP and IVIG, with or without rituximab, are considered standard treatments of acute AMR [5,6], with The Transplantation Society (TTS) recommending that AMR be treated with PP, IVIG, and steroids, followed, if necessary, by adjuvant therapy with rituximab [6,7]. The addition of rituximab to PP plus IVIG has been associated with a more significant reduction in DSA and improved graft survival [5,7,8]. In contrast, a systematic review [9] and another multicenter prospective trial [10] showed that the addition of rituximab had no benefit in patients with AMR. Therefore, despite its widespread use, rituximab-associated B cell depletion has not shown proven benefit in the treatment of AMR [6]. Moreover, targeting of antibody-producing plasma cells with the proteasome inhibitor bortezomib did not improve histology, molecular signatures, or DSA levels in patients with AMR, but had significant toxicity [6]. In contrast, the addition of an alternative irreversible proteasome inhibitor, carfilzomib, to IVIG and PP was found to reduce DSA levels with a favorable toxicity profile [11]. Small studies showed that complement inhibitors had limited efficacy in AMR treatment, with no or only minor clinical effects [11]. Other emerging therapeutic strategies are currently under investigation, such as the anti-interleukin 6 monoclonal antibody tocilizumab, but little progress has been made in the development of effective AMR therapies [6].
High quality data on interventions and drugs for the treatment of AMR are lacking [6,12], and the most appropriate therapeutic strategy remains undetermined. This retrospective study analyzed the effects of treatment with PP and/or IVIG on clinical outcomes, the intensity of anti-HLA antibodies, and the morphological characteristics of biopsy specimens in patients with AMR during the first year after treatment.
Material and Methods
STATISTICAL ANALYSIS:
Numerical data were expressed as the mean±standard deviation, median and range, or number and percentage. Continuous variables were compared by unpaired
Results
Forty-two patients, 22 (52.4%) men and 20 (47.6%) women, of mean age 36.4±12.6 years, fulfilled the inclusion criteria, with 19 (45.2%) having chronic glomerulonephritis of unknown etiology. Analysis of the pre-transplant sensitizing risk factors showed that 22 (52.4%) patients had received a previous blood transfusion and 9 (45%) of the women had previously been pregnant. Eight (19%) patients had preformed DSA, and 16 (38.1%) had preformed non-DSA. Thirty-seven (88.1%) kidneys were from deceased donors, with a mean KDPI index of 43.1±28.8 and a mean 3.9±1.1 mismatches in HLA-A, -B, and -DR. Immunosuppressive induction therapy consisted of monoclonal anti-IL-2 receptor antibodies in 22 (52.4%) patients and anti-thymocyte globulin (mean dose, 5.8±1.2 mg/kg) in 16 (38.1%). AMR treatment consisted of IVIG in 38 (90.5%) patients and PP in 26 (61.9%) (Table 1). Thirteen patients (31.0%) received only IVIG 2 g/kg, and 3 (7.1%) were treated with thymoglobulin or adjustment of maintenance immunosuppressive therapy.
AMR was diagnosed a median 27.2 months (range 0.3 to 213 months) after transplantation. Proteinuria was observed in 36 (85.7%) patients at AMR diagnosis, with mean concentrations of 2.1±3.1 g/g at diagnosis and 1.0±0.9 g/g at the end of follow-up (Table 2). Estimated glomerular filtration rate did not change significantly during follow-up. Eight (19.0%) patients exhibited graft failure, occurring a mean 4.6±3.2 months after AMR treatment, including 5 (62.5%) within the first 6 months. All of these patients had been treated with IVIG and PP. Two presented only with class I DSA (DSA-A 4024 to 13 356 MFI), 3 with only class II DSA (DSA-DQ 3400 to 22 327 MFI), 2 with both DSA-A (3084 to 5000 MFI) and DSA-DQ (7500 to 21 246 MFI) and 1 with both DSA-B (1582 MFI) and DSA-DQ (12 575 MFI). There were no deaths with functioning grafts during the first year.
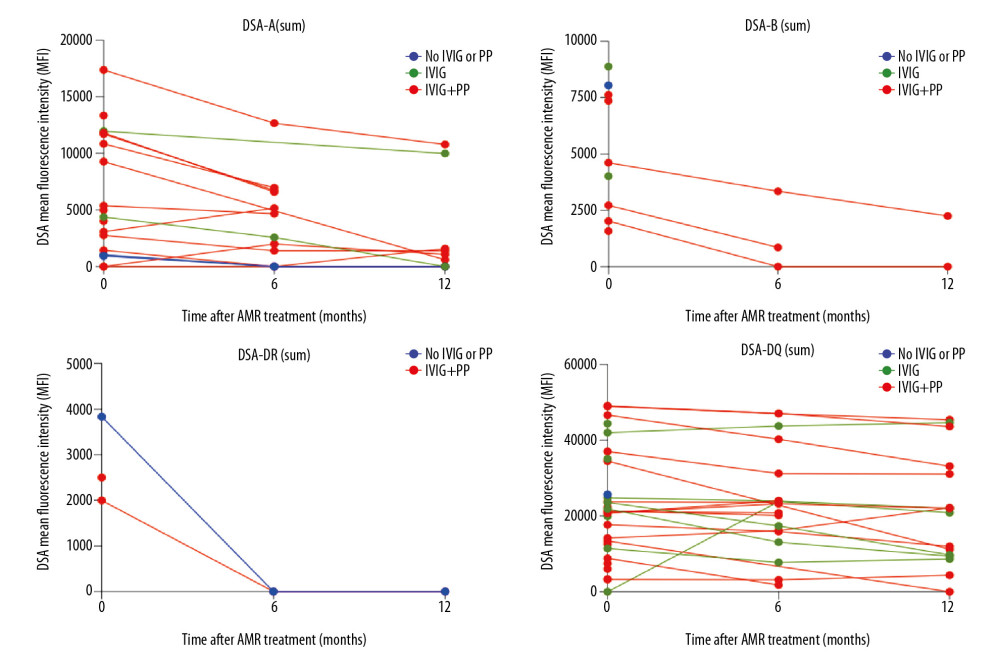
At AMR diagnosis, 18 (42.8%) patients presented with only class II DSA, 11 (26.2%) with only class I, and 13 (31.0%) with both classes I and II. The immunodominant DSA mean fluorescence intensity (MFI) ranged from 945 to 24 883, with a class I DSA sum of 4074±5257 MFI and a class II DSA sum of 16 248±15 612 MFI (Table 2). Six months after treatment, 37 (88.1%) patients had functioning grafts, with a mean class I DSA sum of 2453±3524 MFI and a mean class II DSA sum of 19 780±15 260 MFI. Mean class I PRA decreased from 41.8±33.3% at diagnosis to 21.1±21.7% after 6 months of treatment (p=0.01), whereas class II PRA did not change significantly. The reduction of class I PRA continued throughout follow-up, reaching 16.8±17.6% at the end of the first year (p<0.05). DSA remained detectable in 18 (51.4%) patients with functioning grafts at the end of follow-up; all were treated with IVIG and 11 (61.1%) with PP, and 15 (83.3%) presented with class II DSA (Table 2). DSA-A intensity decreased significantly, from 7198±4982 MFI at AMR diagnosis to 2988±4118 MFI at the end of follow-up (p=0.03), as did DSA-B intensity, from 5254±2761 MFI to 1401±1742 MFI (p=0.04). Only 3 patients were positive for detectable DSA-DR at the diagnosis of AMR, with intensities lower than 3500 MFI, but DSA-DR became undetectable 6 months after treatment. DSA-DQ intensity, which was 23 520±13 597 MFI at AMR diagnosis, was similar, at 21 2878±14 658 MFI, after 12 months (p=0.60).
AMR treatment resulted in a significant reduction in class I DSA (-A and -B) MFI during the first year after therapy (Figure 1). Because most (74.1%) of these patients were treated with both IVIG and PP, it was not possible to compare the effects of these 2 treatments on DSA dynamics. DSA-DR was detectable in only 3 patients with AMR, becoming undetectable after 6 months of follow-up in 2, 1 treated with IVIG and PP and 1 with an adjustment of maintenance immunosuppression. Of the 30 patients who presented with DSA-DQ at AMR diagnosis, 20 (66.7%) received IVIG and PF, 9 (30%) received IVIG alone, and 1 (3.3%) had no specific treatment. None of these patients showed significant changes in DSA MFI throughout follow-up (Figure 1).
Post-treatment graft biopsies showed significant reductions in inflammation score, from 1.0±0.8 to 0.5±0.6 (p<0.05), and in C4d deposit score, from 2.2±1.0 to 1.4±1.2 (p<0.05), without significant changes in other parameters (Table 3). Electron microscopy showed no changes in immune complex deposits, podocyte effacement, or glomerular basement membrane duplication when post-treatment biopsies were compared with biopsies at AMR diagnosis (Table 3). The 23 (54.8%) patients with biopsy-proven AMR more than 2 years after transplantation showed significantly higher scores for glomerular sclerosis, tubulitis (t), peritubular capillaritis (ptc), microvascular inflammation (MVI), and C4d and interstitial fibrosis (ci) when compared with patients with AMR ≤2 years after transplantation (p<0.05); there were no significant differences in other parameters tested. Following treatment, these patients showed reductions in inflammation, tubulitis, and C4d scores (Table 3).
Discussion
The development of an effective treatment protocol for AMR is necessary to improve graft and patient survival, but limited information is available about the immunophenotypic changes that occur after current AMR treatments. PP and immunoadsorption directly remove IgG from serum, but subsequent re-equilibration occurs between the blood and interstitium within 48 h, after which other antibody removal processes may be effective [20]. This may explain the inefficiency of some treatment protocols in the removal of DSA. According to current recommendations for AMR treatment, PP was performed in our patients with a 48-h interval between sessions to increase the efficacy of the method, as the effect of additional therapy remains unclear [6,7].
Another option for AMR treatment is IVIG, possibly due to its ability to neutralize DSAs and inhibit the binding of DSA to target cells in about 80% of patients [1]. The IVIG molecules prevent complement binding or activation, leading to suppression of DSA production [21]. IVIG also inhibits mixed lymphocyte reactions and induces apoptosis, mainly in B cells, reducing the numbers of B cells and monocytes, as well as reducing CD19, CD20, and CD49 expression by B cells, thereby modulating B cell signaling [1]. Multiple retrospective studies have reported the use of IVIG in kidney transplant recipients with AMR, with daily doses ranging from 100–500 mg/kg until response, to a maximum of 2 g/kg, whether administered as a single dose or over several days [2]. Reactions to IVIG can occur within 30 min after the beginning of infusion, and mild reactions are often well managed by reducing the infusion rate [2]. Thrombosis, hemolytic anemia, renal failure, and septic meningitis are possible adverse events of IVIG [2]. In the present study, 2 patients experienced self-limiting headaches during IVIG infusion, with no severe adverse events at follow-up. Although agents directly inhibiting B cell immunity, including rituximab and IVIG, are frequently used to treat AMR, there is limited information on their effectiveness in reversing allograft injury, in reducing DSA generation, and in predicting graft survival [22].
In this series, graft survival was >80% during the first year after AMR diagnosis. No patient with a functioning graft died during this period, and none had severe infection episodes requiring hospitalization after treatment. AMR treatment significantly reduced the MFIs of class I DSAs, without changing DSA-DQ MFI. This finding showed that treatment was ineffective in reducing the MFI of antibodies in patients with AMR related to DSA-DQ, highlighting the need to develop more effective adjunctive therapies for these patients. Treatment also significantly reduced tissue inflammation and C4d scores. The clearance of C4d from the tissue often occurred after the end of antibody response. Moreover, C4d is frequently lost as early as 8 days after treatment [1], similar to our findings. There was no significant change in MVI, measured as the sum of glomerulitis and peritubular capillaritis scores, possibly due to the small sample size and the short follow-up. MVI scores in most of our patients indicated mild to moderate involvement, which may have influenced the response of these parameters to treatment.
AMR was generally more severe 2 years than 1 year after kidney transplantation. The greater severity at 2 years may have been due to the protective effect of immunosuppression on graft inflammation at early diagnosis of AMR. Also, although we did not identify patients with poor adherence to treatment, this possibility cannot be totally excluded, especially in patients later diagnosed with AMR. Treatment was effective in reducing inflammation, tubulitis, and C4d scores in patients with more severe AMR. Despite the absence of cg scores on light microscopy, only 16.7% of our patients with AMR presented with glomerular basement membrane (GBM) duplication on electron microscopy.
Limitations of this study included the lack of protocol biopsy to identify patients with subclinical AMR and the absence of a control biopsy in all treated patients. This study, however, provides important clinical, laboratory, and histological information about the impact of AMR treatment. Another limitation of this study was the absence of additional treatment with rituximab, as this agent has been associated with better outcomes in previous studies.
Conclusions
AMR treatment with IVIG and PP resulted in a significant reduction of class I DSA MFI, without significantly changing DSA-DQ MFI, during the first year of follow-up. The graft biopsies performed after AMR treatment showed significant reductions in inflammation and C4d scores, without improving microvascular inflammation. Treatment did not significantly impair estimated glomerular filtration rate or proteinuria.
Tables
Table 1. General characteristics of the kidney transplant recipients who underwent treatment for biopsy-proven antibody-mediated rejection (AMR).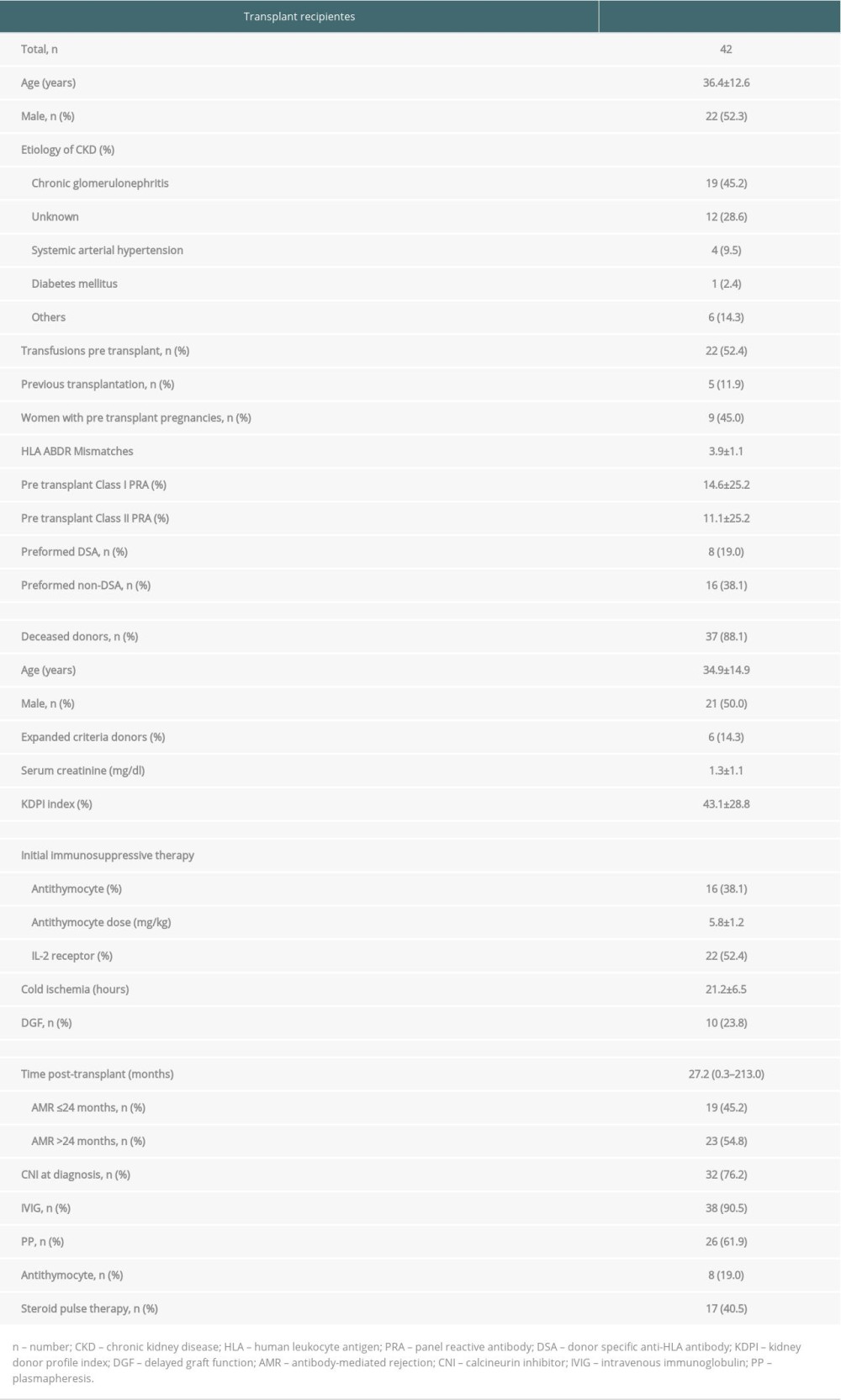 Table 2. Immunopathology and kidney function at diagnosis of antibody-mediated rejection (AMR) and after treatment.
Table 2. Immunopathology and kidney function at diagnosis of antibody-mediated rejection (AMR) and after treatment.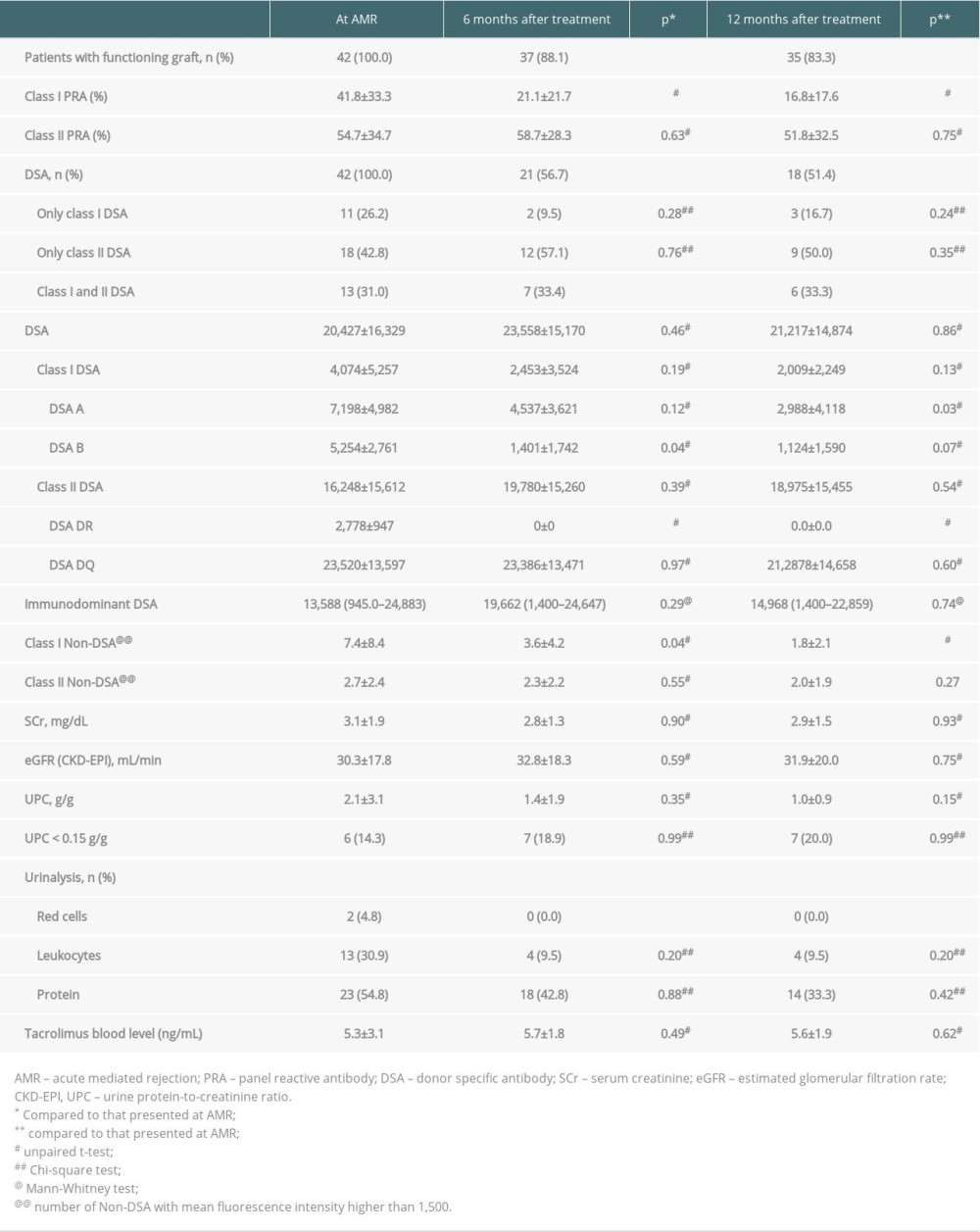 Table 3. Histopathological findings at diagnosis of antibody-mediated rejection (AMR) and after treatment.
Table 3. Histopathological findings at diagnosis of antibody-mediated rejection (AMR) and after treatment.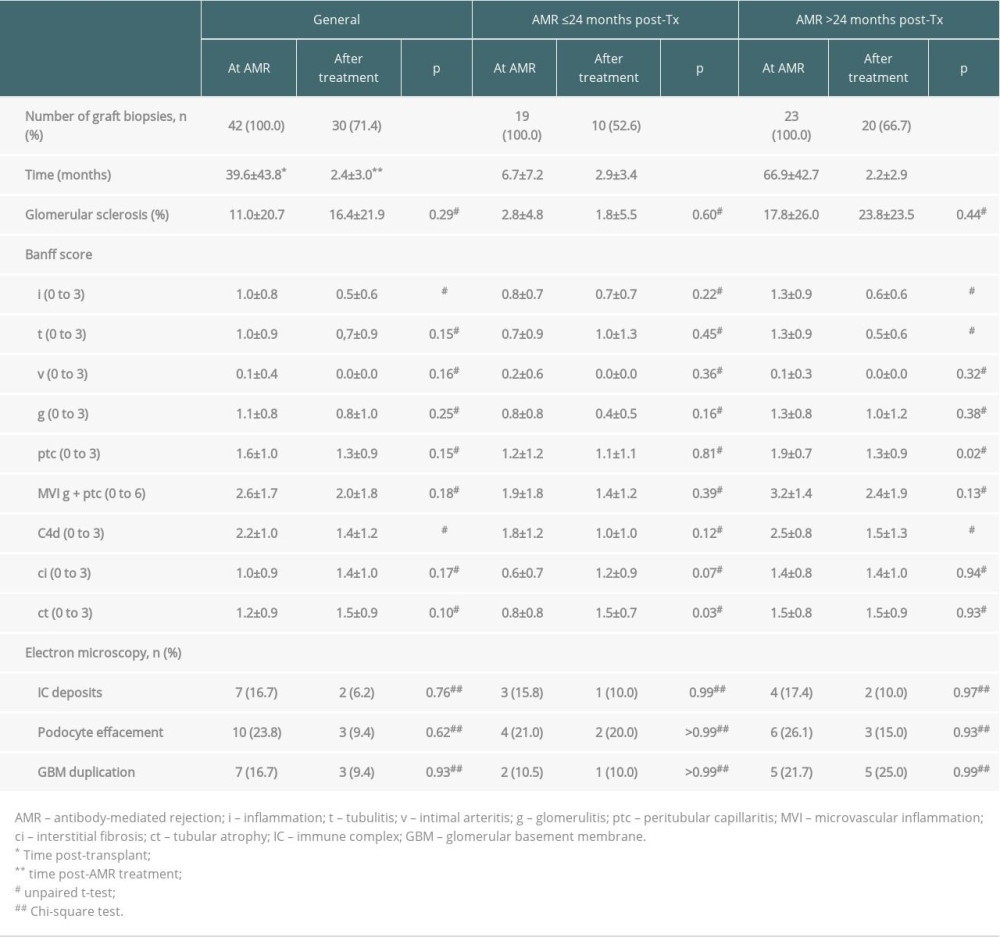
References
1. Colvin RB, Smith RN, Antibody-mediated organ-allograft rejection: Nat Rev Immunol, 2005; 5(10); 807-17
2. Kim M, Martin ST, Townsend KR, Gabardi S, Antibody-mediated rejection in kidney transplantation: A review of pathophysiology, diagnosis, and treatment options: Pharmacotherapy, 2014; 34(7); 733-44
3. Loupy A, Lefaucheur C, Antibody-mediated rejection of solid-organ allografts: N Engl J Med, 2018; 379(12); 1150-60
4. Bartel G, Schwaiger E, Böhmig GA, Prevention and treatment of alloantibody-mediated kidney transplant rejection: Transpl Int, 2011; 24(12); 1142-55
5. Cooper JE, Evaluation and treatment of acute rejection in kidney allografts: Clin J Am Soc Nephrol, 2020; 15(3); 430-38
6. Nickerson PW, What have we learned about how to prevent and treat antibody-mediated rejection in kidney transplantation?: Am J Transplant, 2020; 20(Suppl 4); 12-22
7. Schinstock CA, Mannon RB, Budde K, Recommended treatment for antibody-mediated rejection after kidney transplantation: The 2019 expert consensus from the Transplantion Society Working Group: Transplantation, 2020; 104(5); 911-22
8. Lefaucheur C, Nochy D, Andrade J, Comparison of combination plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection: Am J Transplant, 2009; 9(5); 1099-107
9. Webster AC, Wu S, Tallapragada K, Polyclonal and monoclonal antibodies for treating acute rejection episodes in kidney transplant recipients: Cochrane Database Syst Rev, 2017; 7(7); CD004756
10. Bailly E, Ville S, Blancho G, An extension of the RITUX-ERAH study, multicenter randomized clinical trial comparing rituximab to placebo in acute antibody-mediated rejection after renal transplantation: Transpl Int, 2020; 33(7); 786-95
11. Böhmig GA, Eskandary F, Doberer K, Halloran PF, The therapeutic challenge of late antibody-mediated kidney allograft rejection: Transpl Int, 2019; 32(8); 775-88
12. Moreso F, Crespo M, Ruiz JC, Treatment of chronic antibody mediated rejection with intravenous immunoglobulins and rituximab: A multicenter, prospective, randomized, double-blind clinical trial: Am J Transplant, 2018; 18(4); 927-35
13. de Sousa MV, Gonçalez AC, de Lima Zollner R, Mazzali M: Ann Transplant, 2018; 23; 457-66
14. de Sousa MV, de Lima Zollner R, Mazzali M, Renal transplant patients with preformed anti-HLA antibodies: Early biopsy findings and clinical outcomes: Braz J Nephrol, 2020; 42(2); 201-10
15. Metzger RA, Delmonico FL, Feng S, Expanded criteria donors for kidney transplantation: Am J Transplant, 2003; 3(Suppl 4); 114-25
16. Haas M, Sis B, Racusen LC, Banff 2013 meeting report: inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions: Am J Transplant, 2014; 14(2); 272-83
17. Sampaio WLV, Mazzali M, C4d deposits in borderline rejection: An early marker for chronic renal cysfunction?: Transplant Proc, 2014; 46(6); 1710-12
18. Levey AS, Stevens LA, Schmid CH, A new equation to estimate glomerular filtration rate: Ann Intern Med, 2009; 150(9); 604-12
19. Loupy A, Haas M, Solez K, The Banff 2015 kidney meeting report: Current challenges in rejection classification and prospects for adopting molecular pathology: Am J Transplant, 2017; 17(1); 28-41
20. Montgomery RA, Loupy A, Segev DL, Antibody-mediated rejection: New approaches in prevention and management: Am J Transplant, 2018; 18(Suppl 3); 3-17
21. Zhang R, Donor-specific antibodies in kidney transplant recipients: Clin J Am Soc Nephrol, 2018; 13(1); 182-92
22. Parajuli S, Mandelbrot DA, Muth B, Rituximab and monitoring strategies for late antibody-mediated rejection after kidney transplantation: Transplant Direct, 2017; 3(12); e227
Tables
 Table 1. General characteristics of the kidney transplant recipients who underwent treatment for biopsy-proven antibody-mediated rejection (AMR).
Table 1. General characteristics of the kidney transplant recipients who underwent treatment for biopsy-proven antibody-mediated rejection (AMR). Table 2. Immunopathology and kidney function at diagnosis of antibody-mediated rejection (AMR) and after treatment.
Table 2. Immunopathology and kidney function at diagnosis of antibody-mediated rejection (AMR) and after treatment. Table 3. Histopathological findings at diagnosis of antibody-mediated rejection (AMR) and after treatment.
Table 3. Histopathological findings at diagnosis of antibody-mediated rejection (AMR) and after treatment. Table 1. General characteristics of the kidney transplant recipients who underwent treatment for biopsy-proven antibody-mediated rejection (AMR).
Table 1. General characteristics of the kidney transplant recipients who underwent treatment for biopsy-proven antibody-mediated rejection (AMR). Table 2. Immunopathology and kidney function at diagnosis of antibody-mediated rejection (AMR) and after treatment.
Table 2. Immunopathology and kidney function at diagnosis of antibody-mediated rejection (AMR) and after treatment. Table 3. Histopathological findings at diagnosis of antibody-mediated rejection (AMR) and after treatment.
Table 3. Histopathological findings at diagnosis of antibody-mediated rejection (AMR) and after treatment. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860