13 October 2020: Original Paper
Pediatric Liver Transplantation for Alagille Syndrome: Anesthetic Evaluation and Perioperative Management
Wen-He Yang1ABF, Liang Zhang1ABCDEF*, Fu-Shan Xue1ADG, Azmat Riaz2EF, Zhi-Jun Zhu345ADGDOI: 10.12659/AOT.924282
Ann Transplant 2020; 25:e924282
Abstract
BACKGROUND: Alagille syndrome (AGS) is an autosomal dominant hereditary disorder characterized by identifiable abnormalities in the liver, heart, face, skeleton, and eyes. Recently, liver transplantation (LT) has been proposed as a therapeutic strategy for patients with AGS complicated by end-stage liver disease, but clinical experience in performing anesthesia in LT for AGS is still scarce. We aimed to summarize our preliminary experience in the anesthetic management of LT for AGS in this study.
MATERIAL AND METHODS: We reviewed the cases of 11 patients with AGS who underwent LT from September 2017 to April 2019. Preoperative multi-system comorbidities, intraoperative details, and postoperative outcomes were retrospectively collected and summarized.
RESULTS: Cardiopulmonary abnormalities were common (81.8%) in AGS patients before LT, and the most frequent comorbidity was pulmonary artery stenosis. After careful anesthetic evaluation and perioperative management, all patients survived during the perioperative period without significant cardiovascular complications. However, there was an unexpectedly high prevalence of surgical complications and re-operations in AGS patients compared to biliary atresia recipients (54.5% vs. 22.4%, P=0.031; and 45.5% vs. 15.3%, P=0.028, respectively).
CONCLUSIONS: Perioperative management of LT for AGS patients can be particularly challenging, requiring a full understanding of the pathophysiology, as well as a careful preoperative evaluation of the multi-system comorbidities. The high prevalence of postoperative surgical complications should be a matter of concern.
Keywords: Alagille Syndrome, Anesthesia, Cardiovascular Abnormalities, Liver Transplantation, Pediatrics, Postoperative Complications, Anesthetics, Child, Child, Preschool, End stage liver disease, Infant, Severity of Illness Index
Background
Alagille syndrome (AGS) is an autosomal dominant metabolic disease caused by gene mutations in the NOTCH signaling pathway. AGS is classified into 2 types: Jagged 1 (JAG1) gene mutation is known as AGS type 1, and NOTCH 2 gene mutation is regarded as AGS type 2 [1–3]. The clinical diagnostic criteria for AGS include interlobular bile duct paucity accompanied by at least 3 of the following 5 main features: chronic cholestasis resulting from intrahepatic bile duct hypoplasia, peripheral pulmonary stenosis, dysmorphic facies with a bossed forehead and deep-set eyes, posterior embryotoxon, and butterfly-like vertebrae [2]. Generally, AGS is still a rare disease, with a reported prevalence of 1: 30 000 to 1: 70 000 [2,3].
Chronic cholestasis is the most common manifestation of AGS in infants and young children, and liver transplantation (LT) has been indicated for AGS patients with end-stage liver disease, portal hypertension, and impaired quality of life with growth retardation secondary to serious cholestasis, refractory pruritus, and xanthomatosis. The recently reported 1-year and 5-year survival rates exceeded 80% [4,5]. However, patients with AGS often have varying degrees of cardiovascular, central nervous system, and renal malformations, which can exert adverse effects before, during, and after LT, and possibly influence the prognosis of patients with AGS [4].
There have been several case reports on the anesthetic management for LT in AGS patients with cardiovascular malformations. Adachi et al. suggested strengthening intraoperative monitoring with pulmonary artery catheterization to maintain hemodynamic stability, as well as the guidance of volume therapy [6]. Cheng et al. advocated a judicious preoperative evaluation of the cardiovascular system to screen-out unsuitable candidates for cardiovascular function optimization [7]. Png et al. reported hemodynamic changes in AGS patients during LT, and found that veno-venous bypass (VVB) was helpful in maintaining hemodynamic stability [8]. However, anesthesia experience in LT for AGS patients remains sparse. Therefore, we report our center’s experience in anesthesia management for AGS patients, focusing on preoperative multi-systemic assessment and perioperative management.
Material and Methods
One hundred and seventy-nine children underwent LT between September 2017 and April 2019 at the Beijing Friendship Hospital, Capital Medical University in Beijing, China. The research was approved by the Ethics Committee of Capital Medical University Affiliated Beijing Friendship Hospital (approval number: 2020-P2-044-01). Eleven patients (6.1%) were indicated for AGS, based on either genetic diagnosis or clinical diagnosis. Preoperative recipient variables, donor and liver graft factors, intraoperative data, and postoperative outcomes were analyzed. A whole liver graft was anastomosed using the conventional technique, while a split, reduced-size, or living-related partial liver graft was anastomosed using the modified piggy-back technique. During the anhepatic phase, the inferior vena cava was totally clamped in all cases without the use of VVB. All children were treated according to the standard anesthetic protocol for pediatric LT in our center, and the intraoperative anesthetic data were obtained by reviewing the electronic anesthesia records [9]. Specifically, preoperative cardiovascular assessment and management algorithms of AGS patients were as follows: Echocardiography was initially performed to evaluate cardiac function and screen-out potential cardiopulmonary malformations, and right heart catheterization would be further employed when there were findings of complex congenital heart disease or elevated pulmonary arterial pressure. Patients with mild-to-moderate cardiopulmonary defects and normal cardiac function would proceed to LT without further treatment. For those patients with complex congenital heart disease complicated by cardiac insufficiency, pulmonary hypertension, and right-to-left shunt, a multidisciplinary team (MDT) discussion would be held to determine the appropriateness and timing of LT. Intraoperative arterial blood gas analysis was measured at specific time-points: anesthetic induction, hepatic dissection, anhepatic phase, after reperfusion, and at the end of surgery. Continuous variables are presented as the mean±standard deviation, median (range), or median (interquartile range). Intraoperative variables at different time-points were compared using a repeated-measures analysis of variance.
Results
PREOPERATIVE EVALUATION:
Preoperative assessment of patients includes a physical examination, liver and renal function tests, vitamin and blood glucose levels, abdominal Doppler ultrasonography, upper-abdominal computed tomography angiography, electrocardiogram, echocardiography, arterial blood gas analysis, and chest radiography. Baseline patient and liver graft characteristics are provided in Table 1. All 11 patients were complicated by cholestasis, of whom 9 were misdiagnosed as biliary atresia (BA) and received exploratory laparotomy, laparoscopic exploration, or Kasai portoenterostomy. Nine patients (81.8%) were complicated with cardiovascular malformations identified by echocardiography, with pulmonary artery stenosis (44.4%) as the most common form. However, no patient needed to undergo interventional therapy or cardiovascular surgery, because all patients were New York Heart Association (NYHA) functional class I or II with no signs of severe pulmonary hypertension, right ventricular dysfunction, or right-to-left shunt. The prevalence of posterior embryotoxon, butterfly-like vertebrae, xanthoma, and renal abnormalities was 2 (18.2%), 2 (18.2%), 3 (27.3%), and 3 (27.3%), respectively. Vitamin deficiency (63.6%) was relatively common, and growth failure (72.7%) was a frequent problem in patients with AGS. At the time of surgery, the median (range) age of the patients (8 males and 3 females) was 23.5 (12.0–149.0) months. The median (range) weight and height were 8.5 (6.3–35.8) kg and 82 (66–143) cm, respectively. The mean Child-Turcotte-Pugh (CTP) score and Pediatric End-stage Liver Disease (PELD) score were 7.6±2.1 and 10.8±9.6, respectively. The average graft-to-recipient weight ratio (GRWR) was 2.68±0.91%, and the median (range) graft weight was 267 (200–525) g. Living-related grafts were utilized in 8 of 11 patients, while deceased grafts were employed in 3 patients. The median (range) warm ischemic time and cold ischemic time were 2 (1–5) min and 117 (67–710) min, respectively.
INTRAOPERATIVE DETAILS:
Intraoperative details are shown in Tables 2 and 3. The mean urine output and blood losses were 4.7±1.6 ml/kg/h and 20.8±12.6 ml/kg, respectively. The mean fluid infusion was 11.5±4.1 ml/kg/h, and the average red blood cell infusion was 20.6±15.3 ml/kg. Non-transfusion was accomplished in Case 2, Case 6, and Case 8, fresh frozen plasma was only indicated for Case 1 and Case 4, and no patients required platelet transfusion. Intraoperative changes in serum lactate, potassium, and base excess were similar to those of patients with other indications for LT [10], and no patient received perioperative continuous hemodiafiltration. The incidence of postreperfusion syndrome diagnosed by Aggarwal’s definition was 90.9%, but no patients experienced cardiac arrest or vasoplegic syndrome.
POSTOPERATIVE COMPLICATIONS AND CLINICAL OUTCOMES:
The average durations of Intensive Care Unit (ICU) and hospital stay were 7.3±5.6 days and 35.4±12.7 days, respectively. The mean follow-up time was 24 months (range, 14–34), and patient and graft survival were 100%. The incidence of postoperative surgical complications and re-operations were significantly higher in AGS patients than in BA recipients (54.5% vs. 22.4%, P=0.031; and 45.5% vs. 15.3%, P=0.028, respectively) (for details, see Tables 3, 4). Case 1 developed a hepatic artery thrombus, but did not undergo urgent revascularization or retransplantation after arterial collaterals developed. Bile leakage and hepatic vein anastomotic stricture occurred in Case 2, requiring re-operation on postoperative day (POD) 19 and serial balloon dilatation on POD 30. Two children (Case 7 and Case 9) developed bowel perforation, requiring re-operation for repair. Case 9 developed multiple organ failure due to perforation-related sepsis, and he had needed long-term mechanical ventilation after re-operation; he was eventually moved from the ICU on POD 15 and was discharged on POD 43. Case 3 and Case 11 received exploratory laparotomy due to wound dehiscence and Roux-en-Y anastomotic bleeding, respectively. No patients developed acute heart failure or required inotropic support during the postoperative period. To date, all patients have shown normal physical activities and psychosocial behaviors without deterioration in their cardiac function.
Discussion
In this study, we demonstrate a 100% patient and graft survival in 11 AGS patients. Although the cohort was relatively small, we still found several important findings: (1) Cardiopulmonary malformations were relatively common in AGS patients undergoing LT, among which pulmonary artery stenosis was the most common feature. (2) Mild-to-moderate cardiopulmonary defects did not significantly increase post-LT cardiovascular complications of AGS patients. (3) Compared with pediatric BA recipients, AGS patients had a higher prevalence of postoperative surgical complications.
Several confounding factors have been reported to affect post-transplant outcomes of AGS patients, including surgical technique, anesthetic experience, graft quality, and cardiovascular and other comorbidities associated with the underlying disease. Early and accurate diagnosis of AGS is essential to the treatment and prognosis of patients with AGS. Unfortunately, it is sometimes difficult to make a correct diagnosis because cholestasis in AGS is similar to that in BA [11,12]. In our series, up to 82% of patients were misdiagnosed as BA. Traditionally, the classical diagnosis of AGS requires interlobular bile duct paucity together with at least 3 of the 5 major clinical features, including chronic cholestasis, cardiopulmonary defects, craniofacial abnormalities, posterior embryotoxon, and butterfly vertebrae [4]. Using revised diagnostic criteria, namely a JAG1 mutation or family history of AGS, diagnosis can be done with only 2 of the 5 clinical manifestations [13].
Successful perioperative management of AGS patients relies on effective communication and cooperation between the MDT members consisting of liver transplant surgeons, anesthesiologists, intensivists, pediatricians, geneticists, cardiologists, hepatologists, radiologists, nutritionists, ophthalmologists, orthopedics, and other specialists. Patients should have a comprehensive evaluation of hepatic function, cardiac examination, spinal X-ray, ophthalmologic examination, and renal function. Monitoring and optimizing of growth, development, diet, and nutritional status should be emphasized by MDT members, since growth retardation is known to occur in 50–90% of patients with AGS. Preoperative growth disorders due to fat-soluble vitamin deficiency should be treated with nutritional therapy [14].
Of note, cardiac comorbidities are the leading causes of death for children diagnosed with AGS [15,16], and can seriously affect the outcome of LT for AGS. As a consequence, preoperative assessment and optimization of cardiopulmonary malformations can be of great importance. Common cardiovascular malformations of AGS include peripheral pulmonary stenosis, atrial septal defect, patent foramen ovale, ventricular septal defect, tetralogy of Fallot, and aortic stenosis [2]. In our series, 82% of patients have cardiovascular malformations. After careful evaluation of cardiac function, all patients underwent LT without serious cardiovascular complications. However, it should be noted that AGS patients complicated with severe cardiopulmonary abnormalities were at increased mortality risk after LT [16]. Non-surgical interventional treatments and open-heart surgery may be beneficial in relieving and optimizing perioperative hemodynamics of these patients. Nevertheless, it is still controversial whether cardiac comorbidities should be proactively repaired before LT, and treatment decisions should be determined after a careful discussion by MDT members. Additionally, patients with AGS often have congenital renal abnormalities, intracranial vascular malformations, abdominal aortic aneurysm, intestinal atresia, tracheal and bronchial stenoses, and recurrent fractures, which could lead to fatal postoperative complications and should be carefully screened and evaluated [2,4,14].
The high prevalence of postoperative surgical complications of AGS patients is a new finding of this study. The reason for this remains unclear, but it may be attributed to systemic malnutrition or the history of preoperative abdominal surgery. In this study, 82% of patients were misdiagnosed as BA and underwent exploratory laparotomy, laparoscopic exploration, or Kasai portoenterostomy. Importantly, some patients in this study might have had co-existence of AGS and BA, which was also called BA overlapping AGS [11]. Previous studies have found that patients with AGS who had undergone Kasai procedure could have worse general condition and traumatic biliary tract injuries, which might force them to undergo liver transplants earlier [11,17].
This study has several limitations. First, this study is limited by its retrospective nature and the small sample size. Second, most of the patients did not receive preoperative nutrition therapy before admission to our hospital, suggesting that future research is warranted to observe whether systemic nutrition therapy can minimize postoperative surgical complications. Third, cardiopulmonary malformations of all patients in this study were neither complicated nor serious, and the threshold for correcting cardiopulmonary defects still needs to be clarified in future studies. Finally, no patient in this study was diagnosed as AGS type 2 by preoperative genetic tests, in which type renal dysfunction was more frequent. Renal dysfunction in patients with AGS may complicate perioperative anesthetic management and affect the prognosis of patients. Future studies should focus more on the anesthetic management of LT for AGS type 2 patients.
Conclusions
We report a 100% patient and graft survival in a cohort of 11 pediatric patients with AGS. After careful preoperative evaluation and anesthetic management, mild-to-moderate cardiovascular abnormalities did not carry a high risk for mortality or serious postoperative cardiovascular complications. It has been well-recognized that disease-specific complications improved, and no patients developed cardiovascular complications during the perioperative period. Postoperative surgical complications are relatively frequent, although the underlying causes remain to be elucidated, reflecting the multi-system involvement of the disease itself.
Tables
Table 1. Baseline patient and liver graft characteristics.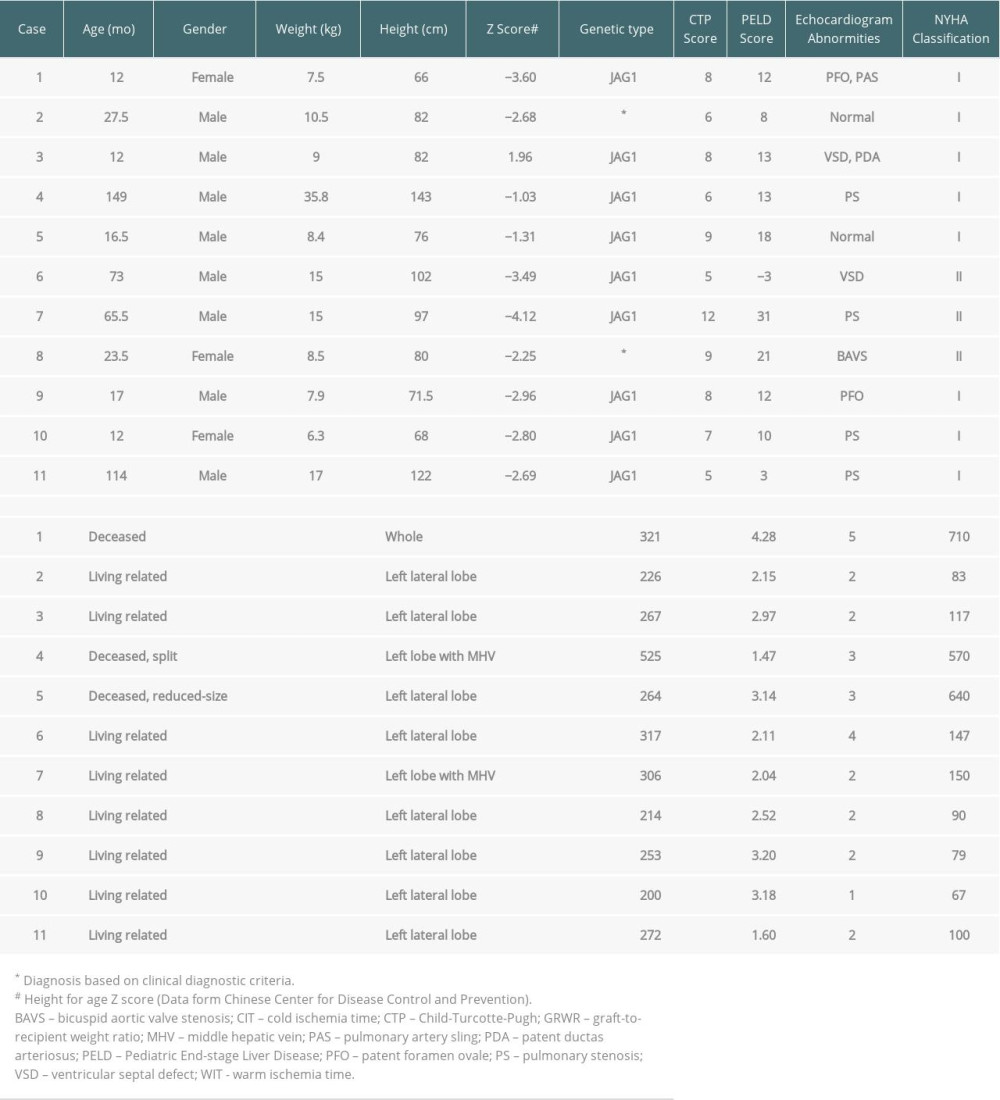 Table 2. Intraoperative hemodynamics, electrolyte, and blood gas measurements.
Table 2. Intraoperative hemodynamics, electrolyte, and blood gas measurements.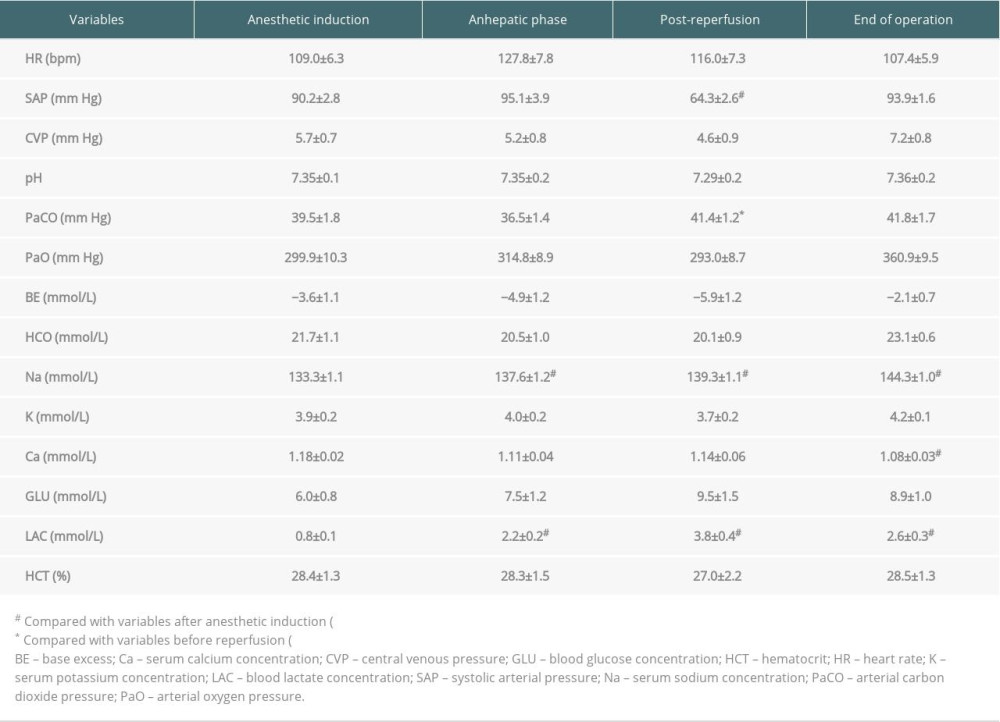 Table 3. Intraoperative management and postoperative outcomes.
Table 3. Intraoperative management and postoperative outcomes.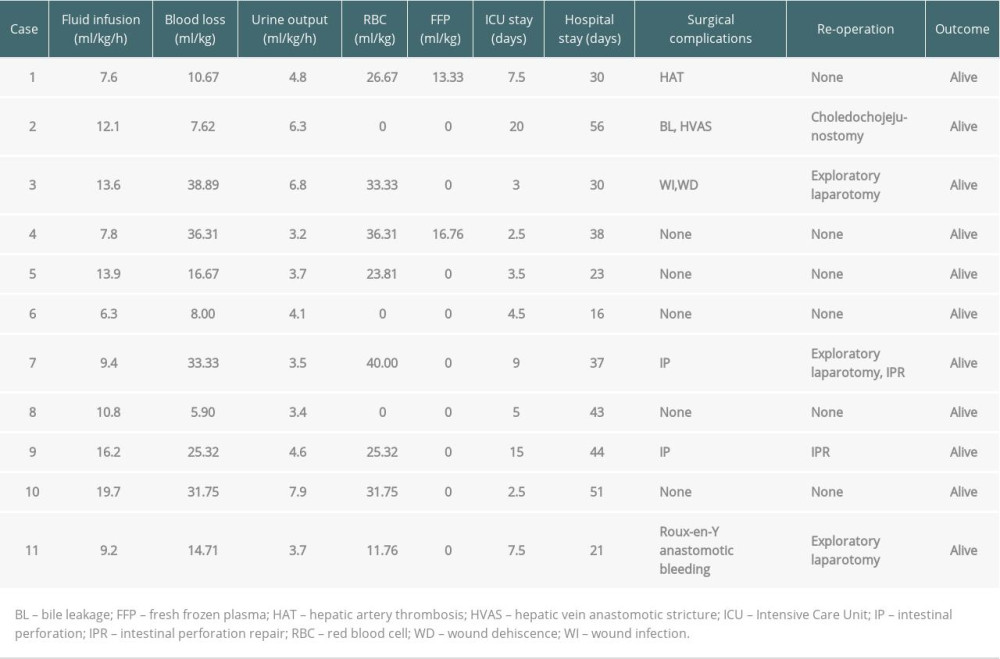 Table 4. Postoperative surgical complications and re-operations in pediatric AGS and BA recipients.
Table 4. Postoperative surgical complications and re-operations in pediatric AGS and BA recipients.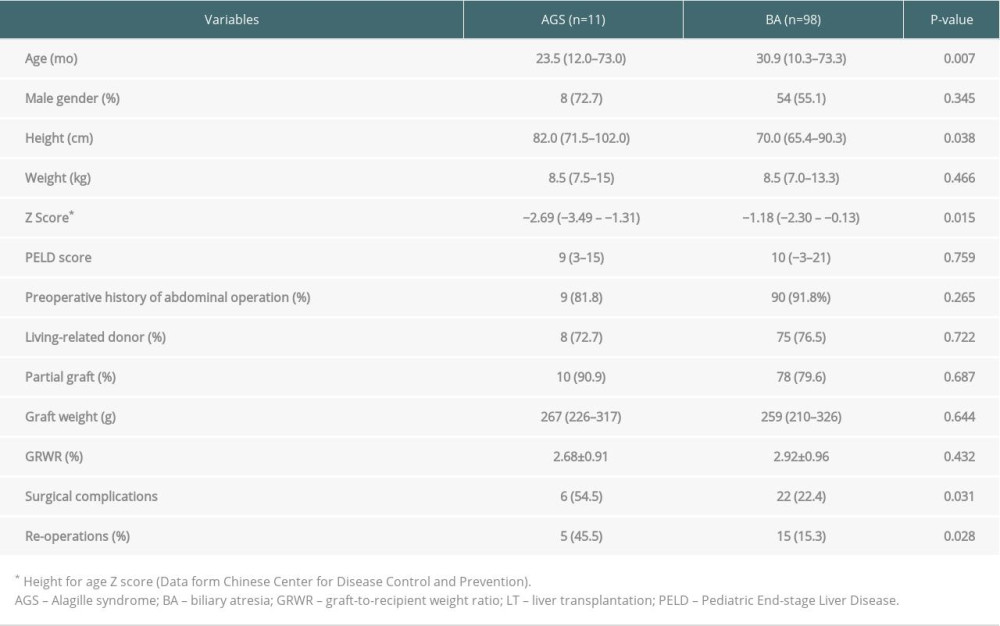
References
1. Kamath BM, Baker A, Houwen R, Systematic review: The epidemiology, natural history, and burden of Alagille syndrome: J Pediatr Gastroenterol Nutr, 2018; 67(2); 148-56
2. Turnpenny PD, Ellard S, Alagille syndrome: Pathogenesis, diagnosis and management: Eur J Hum Genet, 2012; 20(3); 251-57
3. Gilbert MA, Bauer RC, Rajagopalan R, Alagille syndrome mutation update: Comprehensive overview of JAG1 and NOTCH2 mutation frequencies and insight into missense variant classification: Hum Mutat, 2019; 40(12); 2197-220
4. Kamath BM, Yin W, Miller H, Outcomes of liver transplantation for patients with Alagille syndrome: The studies of pediatric liver transplantation experience: Liver Transpl, 2012; 18(8); 940-48
5. Lee CN, Tiao MM, Chen HJ, Characteristics and outcome of liver transplantation in children with Alagille syndrome: A single-center experience: Pediatr Neonatol, 2014; 55(2); 135-38
6. Adachi T, Murakawa M, Uetsuki N, Living related donor liver transplantation in a patient with severe aortic stenosis: Br J Anaesth, 1999; 83(3); 488-90
7. Cheng KW, Huang JJ, Wang CH, Anesthetic management of a patient with Alagille’s syndrome undergoing living donor liver transplantation without blood transfusion: Chang Gung Med J, 2004; 27(6); 449-53
8. Png K, Veyckemans F, De Kock M, Hemodynamic changes in patients with Alagille’s syndrome during orthotopic liver transplantation: Anesth Analg, 1999; 89(5); 1137-42
9. Zhang L, Tian M, Xue F, Diagnosis, incidence, predictors and management of postreperfusion syndrome in pediatric deceased donor liver transplantation: A single-center study: Ann Transplant, 2018; 23; 334-44
10. Zhang L, Zhang Y, Ding GNAnesthetic management of pediatric liver transplantation from living related donors: Journal of Clinical Anesthesiology, 2015; 31(10); 957-61 [in Chinese]
11. Gunadi , Kaneshiro M, Okamoto T, Outcomes of liver transplantation for Alagille syndrome after Kasai portoenterostomy: Alagille Syndrome with agenesis of extrahepatic bile ducts at porta hepatis: J Pediatr Surg, 2019; 54(11); 2387-91
12. Dědič T, Jirsa M, Keil R, Alagille syndrome mimicking biliary atresia in early infancy: PLoS One, 2015; 10(11); e0143939
13. Kamath BM, Spinner NB, Piccoli DA, Alagille Syndrome: Liver disease in children, 2007; 326-45, New York, NY, Cambridge University Press
14. Bonavia A, Pachuski J, Bezinover D, Perioperative anesthetic management of patients having liver transplantation for uncommon conditions: Semin Cardiothorac Vasc Anesth, 2018; 22(2); 197-210
15. Ruth ND, Drury NE, Bennett J, Cardiac and liver disease in children: Implications for management before and after liver transplantation: Liver Transpl, 2020; 26(3); 437-49
16. Smithson S, Hall D, Trachtenberg B, Treatment of cardiovascular complications of Alagille syndrome in clinical optimization for liver transplantation: Int J Cardiol, 2014; 176(2); e37-40
17. Fujishiro J, Suzuki K, Watanabe M, Outcomes of Alagille syndrome following the Kasai operation: A systematic review and meta-analysis: Pediatr Surg Int, 2018; 34(10); 1073-77
Tables
In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








