09 October 2020: Original Paper
Importance of Intraoperative Transfusions of Packed Red Blood Cells and Fresh Frozen Plasma in Liver Transplantation for Hepatocellular Cancer
Łukasz Masior12ABDEF*, Michał Grąt1BCD, Karolina Grąt3B, Maciej Krasnodębski1BF, Karolina M. Wronka4B, Jan Stypułkowski1B, Waldemar Patkowski1B, Mariusz Frączek2B, Marek Krawczyk1BD, Krzysztof Zieniewicz1BDDOI: 10.12659/AOT.923665
Ann Transplant 2020; 25:e923665
Abstract
BACKGROUND: The impact of packed red blood cells (PRBCs) and fresh frozen plasma (FFP) transfusions in patients with hepatocellular cancer (HCC) undergoing liver transplantation has rarely been evaluated. The aim of the current study was to assess the impact of intraoperative transfusions on posttransplant outcomes.
MATERIAL AND METHODS: This retrospective cohort study was based on 229 HCC transplant recipients. The primary outcome measure was 5-year recurrence-free survival. Secondary outcome measures comprised overall and long-term survival at 5 years and 90-day mortality. Cox proportional hazard models and logistic regression were used to assess risk factors.
RESULTS: After adjustment for potential confounders, no association was found with respect to tumor recurrence for PRBCs (P=0.368) or FFP (P=0.081) transfusions. Similarly, PRBC transfusion (P=0.623) and FFP transfusion (P=0.460) had no impact on survival between 90 days and 5 years. PRBC transfusion increased the risk of 90-day mortality (P=0.005), while FFP transfusion was associated with a lower risk (P=0.036).
CONCLUSIONS: Intraoperative transfusions of blood products does not impair recurrence-free and long-term survival of patients with HCC undergoing liver transplantation. Intraoperative PRBC transfusion increases the risk of early mortality, whereas adequate supplementation of FFP plays a protective role.
Keywords: Blood Transfusion, Carcinoma, Hepatocellular, Liver Neoplasms, Liver Transplantation, Blood Component Transfusion, Erythrocytes, Plasma, young adult
Background
Hepatocellular cancer (HCC) is the sixth most common malignancy worldwide and the second most common cause of cancer-related deaths [1–3]. Liver transplantation remains the most effective treatment for curing both the cancer and the pathology underlying its development [4,5].
The current benchmark criteria for transplantation (Milan criteria) are based solely on morphological features and yield good results [6,7]. However, numerous studies show that the number and size of tumors are not sufficient for assessing the prognosis of patients with HCC [8–11]. In addition, the transfusion of blood products has been found to have a detrimental effect in various types of cancer in terms of early mortality, long-term survival, and recurrence [12–15]. The main factor is immune based and has been termed transfusion-related immunomodulation [16].
The effects of transfusions with packed red blood cells (PRBCs) and fresh frozen plasma (FFP) in patients with HCC undergoing liver transplantation have rarely been evaluated. The aim of the present study was to assess whether such transfusions affect the outcomes of HCC patients treated with liver transplantation.
Material and Methods
This retrospective cohort study included 229 patients with HCC who were treated with liver transplantation between December 1994 and December 2014 at the Department of General, Transplant and Liver Surgery of the Medical University of Warsaw. Only patients with classic HCC were included. The study protocol was approved by the Ethics Committee of the Medical University of Warsaw.
All clinical variables were extracted from a database of patients with primary and secondary liver tumors at our center. This information is prospectively collected, and data on the number of PRBC and FFP units transfused during each patient’s procedure were taken from the anesthesia cards. The transplantation techniques and the regimens used for immunosuppressive treatment were previously described [17,18].
Qualitative variables are presented as numbers and percentages, and quantitative variables as medians and ranges. Characteristics of the study group included the main analyzed variables (intraoperative transfusion of PRBCs and FFP), oncological variables, and non-oncological variables. The characteristics of 2 groups of patients within and outside Milan criteria were compared. The Fisher’s exact test was used to compare the qualitative variables, and the Mann-Whitney U test was used to assess quantitative variables.
The primary outcome measure was 5-year recurrence-free survival, and the secondary outcome measures were 5-year overall survival, long-term survival, and 90-day postoperative mortality. Long-term survival specifically referred to patients who survived beyond 90 days after surgery. To assess the relevant risk factors, the Cox proportional hazards model and logistic regression were used. Multivariate models were created using the stepwise forward method. The level of statistical significance for including variables in the model was set at
Survival curves were calculated using the Kaplan-Meier method. Overall survival was calculated from the date of transplantation until patient death irrespective of cause and censored at 5 years after transplantation or last follow-up visit. Accordingly, recurrence-free survival was calculated from the date of transplantation until the first documented recurrence and censored at 5 years after transplantation or last follow-up visit. Comparison of survival curves was made using a log-rank test. Confidence intervals (CIs) were given with 95% limits. The statistical significance limit was set at 0.05. All statistical analyses were carried out using the Statistica 12 statistical software (StatSoft Inc).
Results
The studied cohort predominantly contained men (65.5% of all patients), and the median age of recipients was 57 years (range 20–71 years). Their median MELD (model for end-stage liver disease) score was 11 points (range 6–40 points). The median number of tumors in the explanted liver was 1, and the median size of the largest lesion was 30 mm. One hundred of the 229 patients underwent neoadjuvant therapy before transplantation (43.7% of patients). The most commonly used method was transarterial chemoembolization (TACE), which was applied in 77 patients (33.6%). The detailed characteristics of the study group are provided in Table 1. In a comparison of patients who met and did not meet the Milan criteria, significant differences were observed only for the majority of oncological variables (Table 2).
The median follow-up was 35.7 months, and the 90-day mortality rate was 8.7% (20 patients). The 5-year overall survival in the whole group was 68.6%, with recurrence-free survival reaching 78.7%. The 5-year overall survival of patients fulfilling Milan criteria (71.4%) was better than that of the patient who did not meet the criteria (65.4%); however, the difference was not statistically significant (
In multivariable analyses, recurrence-free survival was significantly influenced exclusively by oncological measures, such as α-fetoprotein concentration (
After exclusion of early deaths, risk factors were analyzed among patients who survived more than 90 days after transplantation. Neither PRBC transfusion (HR 0.98 for 1 unit, 95% CI 0.92–1.05;
In groups of patients stratified by the fulfilment of Milan criteria, we observed no negative impact of transfusions on overall survival and recurrence-free survival. This effect was not demonstrated for either PRBCs or FFP (Tables 4, 5).
Discussion
Based on the data presented, blood product transfusions did not have a significant impact on recurrence-free survival in patients with HCC undergoing liver transplantation. This lack of significance did not change with further analysis based on whether or not patients met the eligibility criteria (i.e., Milan, UCSF, and Up-to-7). Zhou et al. [19] found similar results in a cohort of patients treated by liver resection. In that study, blood transfusion negatively affected overall survival but had no impact on recurrence, mirroring findings from other centers [20–22].
However, transfusion of PRBCs was an important factor worsening the 5-year overall survival in our cohort, which is in line with results of previous studies [19,23–25]. Despite many reports about the adverse effects of blood transfusions in patients treated for HCC, findings are not consistent. In an analysis of a group of patients treated by liver resection, Yang et al. [26] found no significant effect of blood transfusions on long-term and recurrence-free survival. In addition, we did not observe that intraoperative transfusion of FFP had an adverse effect on overall survival. Similar results were obtained by Kaibori et al. [27] for a group of 410 patients undergoing liver resection due to HCC.
It should be emphasized that analyses of the influence of blood transfusions in patients with HCC are dominated by individuals who are undergoing liver resection [23,25,26,28]. Data on patients treated with liver transplantation are scarce. Liu et al. [29] showed that intraoperative blood loss, rather than intraoperative transfusion of PBRCs, was an important risk factor for a cancer recurrence after liver transplantation. In another study from the same center, an analogous relationship was demonstrated for intraoperative blood loss and overall survival [30]. Because of the equivocal reports about the significance of intraoperative transfusions on long-term outcomes, we analyzed the patients who died in the early posttransplant period (up to 90 days) and those who survived beyond this point separately. The analyses did not confirm adverse effects of PBRC and FFP transfusions on long-term survival. The only significant factor was the number of tumors. Next to tumor size, the number of tumors is considered to be one of the main factors determining long-term survival. These 2 parameters are the main components of most current eligibility criteria for liver transplantation in patients with HCC. Therefore, the adverse effect of the number of tumors on the prognosis is not surprising and confirms the observation of other centers [9,10,31–34].
Data from the National Program for the Improvement of Quality in Surgery showed that intraoperative transfusion of even 1 unit of blood significantly increased the risk of both 30-day mortality and postoperative complications [35]. Subsequent analysis of 500 000 patients from this registry showed that intraoperative blood transfusion was a significant risk factor for perioperative systemic inflammatory response syndrome, which increased mortality over 13-fold (13.5%
Excessive blood loss and hemostatic disorders are phenomena that often occur during liver transplantation. In particular, their dynamics resemble massive traumatic hemorrhage [37]. In a group of patients with massive traumatic hemorrhage, dynamic use of blood products (RPBCs, FFP, and platelets at a ratio of 1: 1: 1) led to a significant reduction in mortality through rapid correction of coagulopathy [38]. In our center, an aggressive strategy of supplementing FFP during liver transplantation was used. The median number of PRBC and FFP units transfused intraoperatively was 3 and 6, respectively. This approach may explain the protective effect of FFP transfusion on the early results of liver transplantation in our cohort. Another potential explanation is the beneficial effect of FFP on increasing the opsonic activity, which may reduce the risk of infection in the postoperative period [27].
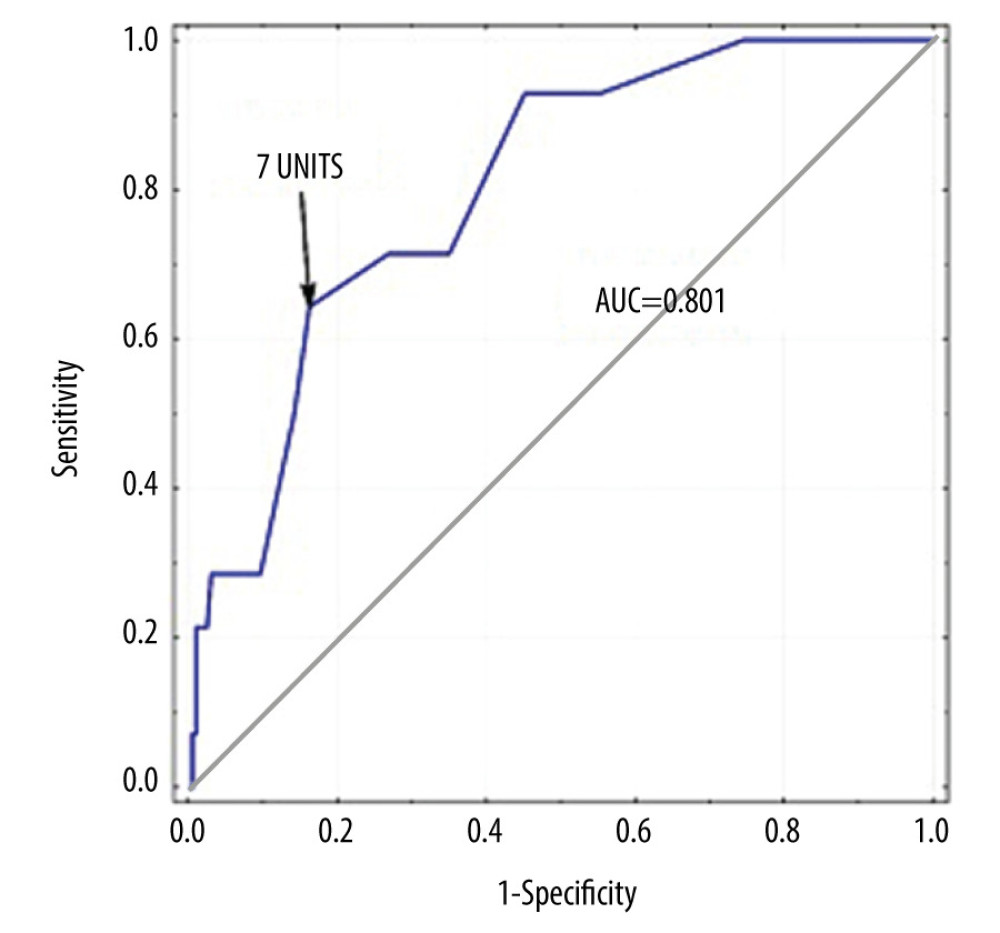
Data from the literature show that the impact of transfusions is associated not only with their use, but is also directly proportional to the number of transfused units [35]. Thus, we conducted a separate analysis to explore the potential influence of this variable. In the prediction of early deaths, the transfusion of at least 7 units of PRBCs had a sensitivity of 64.3% and a specificity of 83.8% (ROC=.801). This finding was confirmed by differences in postoperative mortality, which was as high as 22% among patients who had received 7 or more units compared with 2.9% mortality among patients who received fewer units. Considering the median number of units of PBRCs transfused in our group, which was 3, the relationship seems natural, especially given that even 1 intraoperatively transfused unit increases the risk of postoperative death [35]. However, in some studies only the transfusion of more than 28 units of PRBC correlated well with an increase in mortality [39]. Based on the tendency to gradually reduce transfusion rates during liver transplantation, that value should be considered to be extremely high. The threshold of 7 units that we set appears to have more practical value, but it should be noted that the number could vary between centers.
The analysis of Kaplan-Meier curves also provided an interesting observation. Despite no effect of PRBC transfusion on the long-term survival of patients in multivariable analyses, the comparison of survival curves of patients from high- and low-risk groups revealed a statistical tendency toward worse 5-year long-term survival in patients with more than 4 units transfused. Massicotte et al. [40] showed that 1-year survival after liver transplantation was significantly worse if more than 4 units of PRBCs were transfused, with a decrease from 86.6% to 62.5%. Ramos et al. [41] reported similar conclusions from their study cohort, with intraoperative transfusion of 6 units of PRBCs reducing the 3-year survival from 82% to 58%. Therefore, massive intraoperative blood transfusion should be considered to have a potentially unfavorable effect on long-term survival in patients undergoing liver transplantation due to HCC.
Owing to its retrospective nature, the present study is subject to certain limitations. Because no clear thresholds for intraoperative blood product transfusions have been established, we were not able to exclude the importance of, for example, differences in management between anesthetic teams that could affect the number of transfused units during liver transplantation. However, our results were not affected by this factor because we analyzed the transfusions themselves not the specific reasons behind them. Moreover, any future prospective randomized trial is highly unlikely for ethical reasons. Therefore, we suggest that intraoperative transfusion be viewed as a parameter reflecting the difficulty of a procedure and indirectly as the amount of blood loss. However, given the biological activity of blood products, a causal relationship of transfusion with effects is possible.
Conclusions
The analyses performed in the current study did not confirm that intraoperative transfusion of blood products significantly increases the risk of HCC recurrence in patients treated with liver transplantation. An effect on long-term survival was also not demonstrated, although it cannot be ruled out for patients requiring massive transfusion during the procedure. Intraoperative PRBC transfusion and the associated increased risk of early mortality likely reflect the magnitude of the surgery and the degree of blood loss, although a direct relationship between transfusion and an increased risk of 90-day mortality is also possible. Adequate supplementation of FFP during liver transplantation is associated with a lower risk of perioperative mortality.
Tables
Table 1. Characteristics of the study cohort.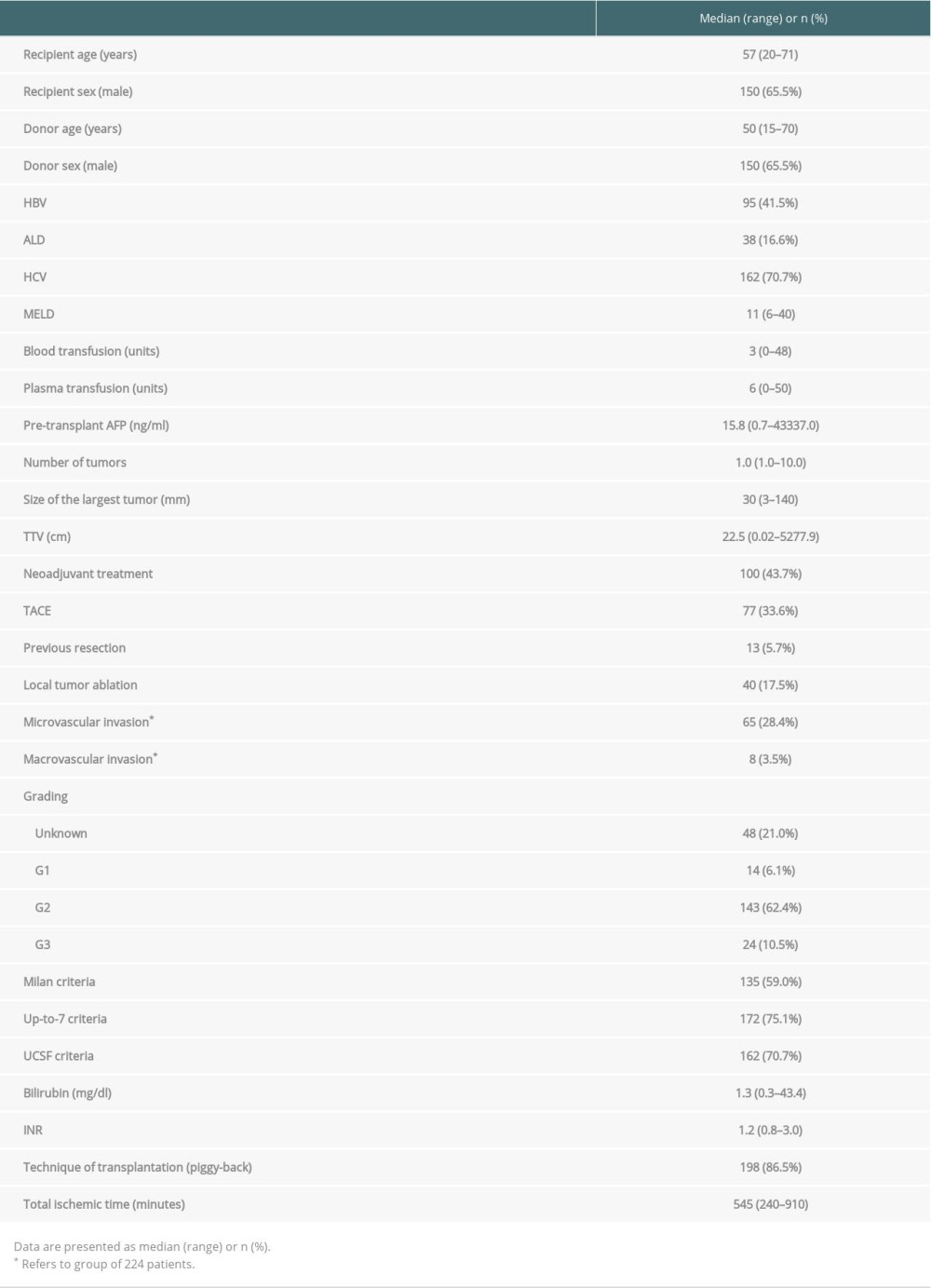 Table 2. Comparision of patients within and beyond Milan criteria.
Table 2. Comparision of patients within and beyond Milan criteria.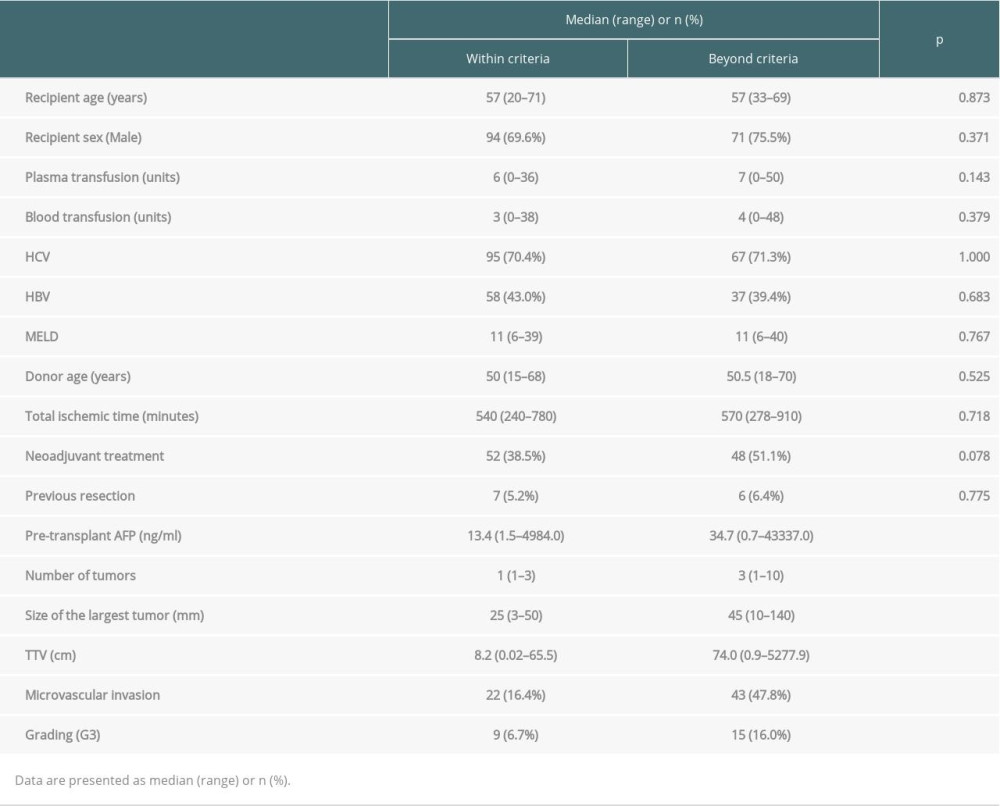 Table 3. Multivariable analyses of factors related to recurrence-free survival.
Table 3. Multivariable analyses of factors related to recurrence-free survival.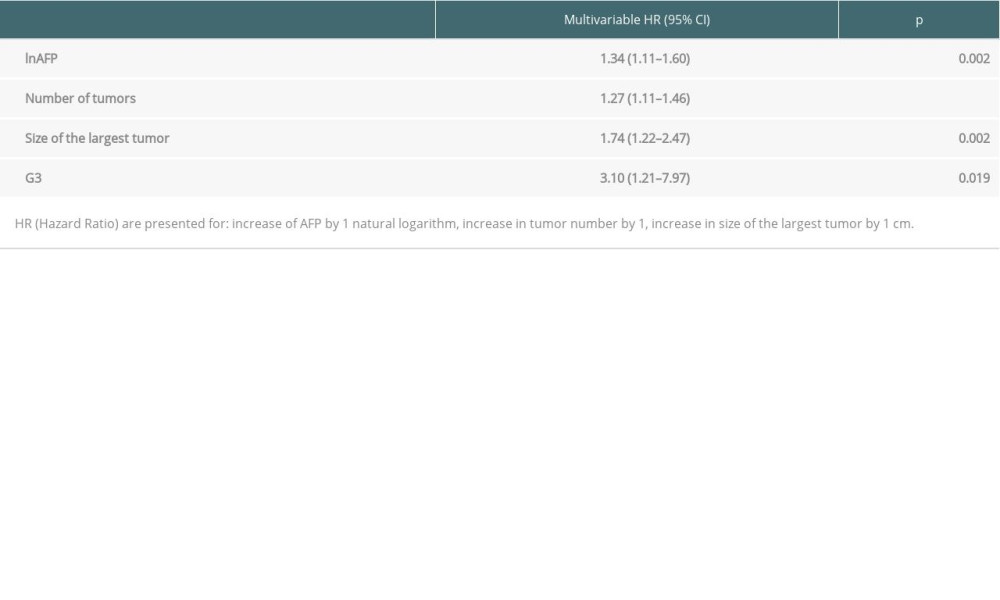 Table 4. Impact of transfusions on overall survival based on certain transplant criteria.
Table 4. Impact of transfusions on overall survival based on certain transplant criteria.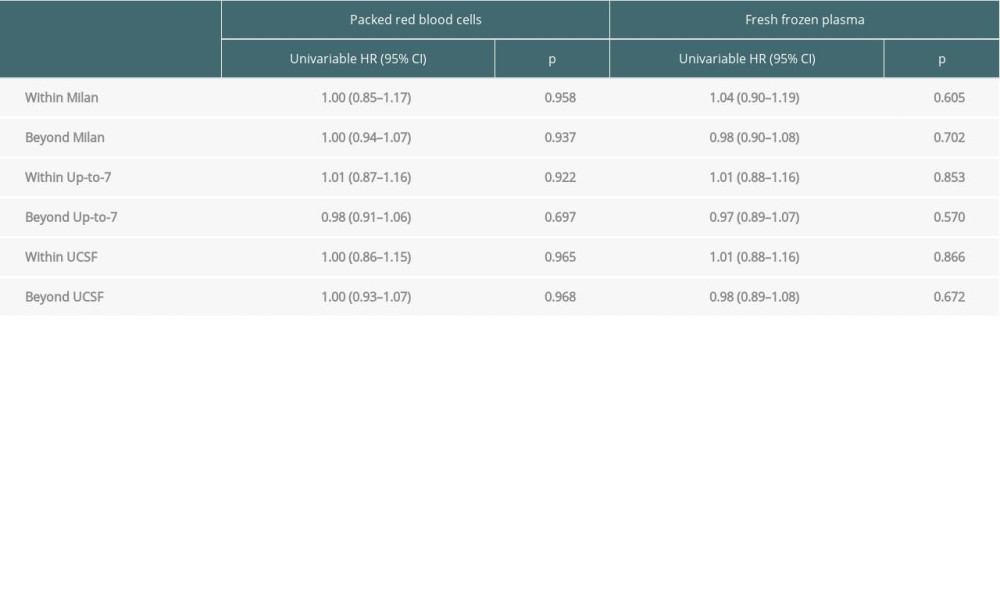 Table 5. Impact of transfusions on recurrence-free survival based on certain transplant criteria.
Table 5. Impact of transfusions on recurrence-free survival based on certain transplant criteria.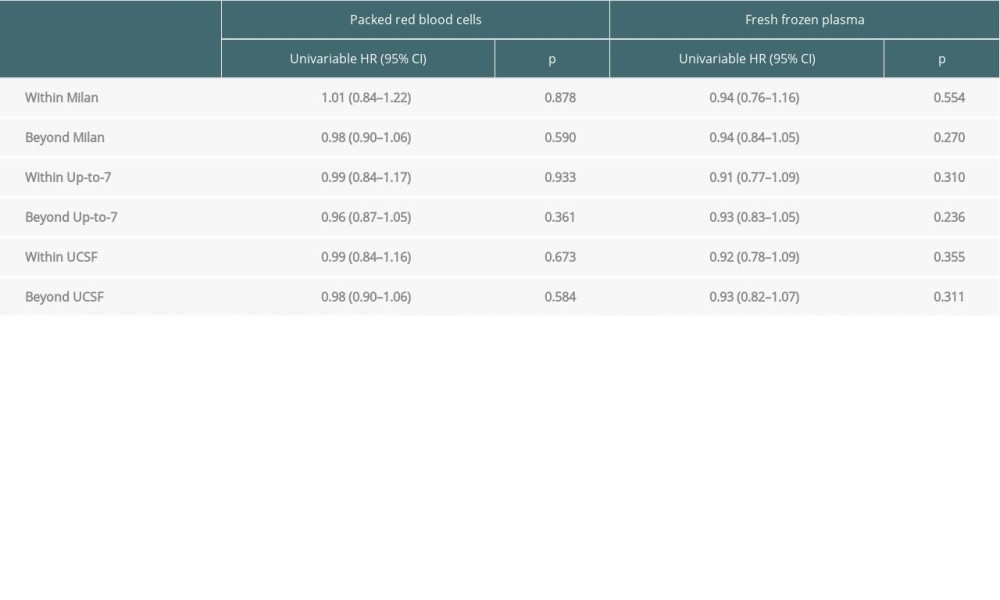
References
1. Venook AP, Papandreou C, Furuse J, de Guevara LL, The incidence and epidemiology of hepatocellular carcinoma: A global and regional perspective: Oncologist, 2010; 15(Suppl 4); 5-13
2. McGlynn KA, Petrick JL, London WT, Global epidemiology of hepatocellular carcinoma: An emphasis on demographic and regional variability: Clin Liver Dis, 2015; 19; 223-38
3. Mak LY, Cruz-Ramón V, Chinchilla-López P, Global epidemiology, prevention, and management of hepatocellular carcinoma: Am Soc Clin Oncol Educ Book, 2018; 38; 262-79
4. Lai Q, Vitale A, Iesari SEuropean Hepatocellular Cancer Liver Transplant Study Group, Intention-to-treat survival benefit of liver transplantation in patients with hepatocellular cancer: Hepatology, 2017; 66; 1910-1919
5. Pinna AD, Yang T, Mazzaferro V, Liver transplantation and hepatic resection can achieve cure for hepatocellular carcinoma: Ann Surg, 2018; 268; 868-75
6. Mazzaferro V, Regalia E, Doci R, Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis: N Engl J Med, 1996; 334; 693-99
7. Clavien PA, Lesurtel M, Bossuyt PMOLT for HCC Consensus Group, Recommendations for liver transplantation for hepatocellular carcinoma: An international consensus conference report: Lancet Oncol, 2012; 13; e11-22
8. Costentin CE, Bababekov YJ, Zhu AX, Yeh H, Is it time to reconsider the Milan Criteria for selecting patients with hepatocellular carcinoma for deceased-donor liver transplantation?: Hepatology, 2019; 69; 1324-36
9. Grąt M, Wronka KM, Stypułkowski J, The Warsaw proposal for the use of extended selection criteria in liver transplantation for hepatocellular cancer: Ann Surg Oncol, 2017; 24; 526-34
10. Halazun KJ, Tabrizian P, Najjar M, Is it time to abandon the Milan Criteria? Results of a bicoastal US collaboration to redefine hepatocellular carcinoma liver transplantation selection policies: Ann Surg, 2018; 268; 690-99
11. Giard JM, Mehta N, Dodge JL, Alpha-fetoprotein slope >7.5 ng/mL per month predicts microvascular invasion and tumor recurrence after liver transplantation for hepatocellular carcinoma: Transplantation, 2018; 102; 816-22
12. Wu HL, Tai YH, Lin SP, The impact of blood transfusion on recurrence and mortality following colorectal cancer resection: A propensity score analysis of 4,030 patients: Sci Rep, 2018; 8; 13345
13. Goubran HA, Elemary M, Radosevich M, Impact of transfusion on cancer growth and outcome: Cancer Growth Metastasis, 2016; 9; 1-8
14. Mavros MN, Xu L, Maqsood H, Perioperative blood transfusion and the prognosis of pancreatic cancer surgery: Systematic review and meta-analysis: Ann Surg Oncol, 2015; 22; 4382-91
15. Shiba H, Misawa T, Fujiwara Y, Negative impact of fresh-frozen plasma transfusion on prognosis of pancreatic ductal adenocarcinoma after pancreatic resection: Anticancer Res, 2013; 33; 4041-47
16. Vamvakas EC, Blajchman MA, Transfusion-related immunomodulation (TRIM): An update: Blood Rev, 2007; 21; 327-48
17. Grąt M, Kornasiewicz O, Lewandowski Z, The impact of surgical technique on the results of liver transplantation in patients with hepatocellular carcinoma: Ann Transplant, 2013; 18; 448-59
18. Krawczyk M, Grąt M, Barski K, 1000 liver transplantations at the Department of General, Transplant and Liver Surgery, Medical University of Warsaw – analysis of indications and results: Pol Przegl Chir, 2012; 84; 304-12
19. Zhou L, Rui JA, Wang SB, Outcomes and prognostic factors of cirrhotic patients with hepatocellular carcinoma after radicalmajor hepatectomy: World J Surg, 2007; 31; 1782-87
20. Wada H, Eguchi H, Nagano H, Perioperative allogenic blood transfusion is a poor prognostic factor after hepatocellularcarcinoma surgery: A multi-center analysis: Surg Today, 2018; 48; 73-79
21. Ercolani G, Grazi GL, Ravaioli M, Liver resection for hepatocellular carcinoma on cirrhosis: Univariate and multivariate analysis of risk factors for intrahepatic recurrence: Ann Surg, 2003; 237; 536-43
22. Kwon AH, Matsui Y, Kamiyama Y, Perioperative blood transfusion in hepatocellular carcinomas: Influence of immunologic profile and recurrence free survival: Cancer, 2001; 91; 771-78
23. Liu L, Wang Z, Jiang S, Perioperative allogenenic blood transfusion is associated with worse clinical outcomes for hepatocellular carcinoma: A meta-analysis: PLoS One, 2013; 8; 64261
24. Poon RT, Fan ST, Lo CM, Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases. Analysis of 1222 consecutive patients from a prospective database: Ann Surg, 2004; 240; 698-710
25. Franssen B, Jibara G, Tabrizian P, Actual 10-year survival following hepatectomy for hepatocellular carcinoma: HPB (Oxford), 2014; 16; 830-35
26. Yang T, Lu JH, Lau WY, Perioperative blood transfusion does not influence recurrence-free and overall survivals after curative resection for hepatocellular carcinoma. A propensity score matching analysis: J Hepatol, 2016; 64; 583-93
27. Kaibori M, Saito T, Matsui K, Impact of fresh frozen plasma on hepatectomy for hepatocellular carcinoma: Anticancer Res, 2008; 28; 1749-56
28. Zhou L, Rui JA, Wang SB, Outcomes and prognostic factors of cirrhotic patients with hepatocellular carcinoma after radical major hepatectomy: World J Surg, 2007; 31; 1782-8
29. Liu B, Teng F, Fu H, Excessive intraoperative blood loss independently predicts recurrence of hepatocellular carcinoma after liver transplantation: BMC Gastroenterol, 2015; 15; 138
30. Teng F, Han QC, Ding GS, Validation of a criteria-specific long-term survival prediction model for hepatocellular carcinoma patients after liver transplantation: Sci Rep, 2015; 5; 11733
31. Zaydfudim VM, Vachharajani N, Klintmalm GB, Liver resection and transplantation for patients with hepatocellular carcinoma beyond Milan Criteria: Ann Surg, 2016; 264; 650-58
32. Fan J, Yang GS, Fu ZR, Liver transplantation outcomes in 1,078 hepatocellular carcinoma patients: A multi-center experience in Shanghai, China: J Cancer Res Clin Oncol, 2009; 135; 1403-12
33. Grąt M, Stypułkowski J, Patkowski W, Challenging the principle of utility as a barrier for wider use of liver transplantation for hepatocellular cancer: Ann Surg Oncol, 2017; 24; 3188-95
34. Mazzaferro V, Sposito C, Zhou J, Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma: Gastroenterology, 2018; 154; 128-39
35. Bernard AC, Davenport DL, Chang PK, Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery patients: J Am Coll Surg, 2009; 208; 931-37 discussion 938–39
36. Ferraris VA, Ballert EQ, Mahan A, The relationship between intraoperative blood transfusion and postoperative systemic inflammatory response syndrome: Am J Surg, 2013; 205; 457-65
37. de Boer MT, Molenaar IQ, Hendriks HG, Minimizing blood loss in liver transplantation: Progress through research and evolution of techniques: Dig Surg, 2005; 22; 265-75
38. Stensballe J, Henriksen HH, Johansson PI, Early haemorrhage control and management of trauma-induced coagulopathy: The importance of goal-directed therapy: Curr Opin Crit Care, 2017; 23; 503-10
39. Rana A, Petrowsky H, Hong JC, Blood transfusion requirement during liver transplantation is an important risk factor for mortality: J Am Coll Surg, 2013; 216; 902-7
40. Massicotte L, Sassine MP, Lenis S, Survival rate changes with transfusion of blood products during liver transplantation: Can J Anesth, 2005; 52; 148-55
41. Ramos E, Dalmau A, Sabate A, Intraoperative red blood cell transfusion in liver transplantation: Influence on patient outcome, prediction of requirements, and measures to reduce them: Liver Transpl, 2003; 9; 1320-27
Figures
Tables
 Table 1. Characteristics of the study cohort.
Table 1. Characteristics of the study cohort. Table 2. Comparision of patients within and beyond Milan criteria.
Table 2. Comparision of patients within and beyond Milan criteria. Table 3. Multivariable analyses of factors related to recurrence-free survival.
Table 3. Multivariable analyses of factors related to recurrence-free survival. Table 4. Impact of transfusions on overall survival based on certain transplant criteria.
Table 4. Impact of transfusions on overall survival based on certain transplant criteria. Table 5. Impact of transfusions on recurrence-free survival based on certain transplant criteria.
Table 5. Impact of transfusions on recurrence-free survival based on certain transplant criteria. Table 1. Characteristics of the study cohort.
Table 1. Characteristics of the study cohort. Table 2. Comparision of patients within and beyond Milan criteria.
Table 2. Comparision of patients within and beyond Milan criteria. Table 3. Multivariable analyses of factors related to recurrence-free survival.
Table 3. Multivariable analyses of factors related to recurrence-free survival. Table 4. Impact of transfusions on overall survival based on certain transplant criteria.
Table 4. Impact of transfusions on overall survival based on certain transplant criteria. Table 5. Impact of transfusions on recurrence-free survival based on certain transplant criteria.
Table 5. Impact of transfusions on recurrence-free survival based on certain transplant criteria. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860