18 September 2020: Original Paper
ABO-Incompatible Living Donor Liver Transplantation with Reduced Rituximab Dose: A Retrospective Analysis of 65 Patients – Can We Fast-Track Liver Transplant Surgery and Improve Long-Term Survival?
Shih-Chao Hsu123ABCDEF, Ashok Thorat13ABCEF, Long-Bin Jeng143ABCDEF*, Ping-Chun Li135ADF, Te-Hung Chen123BCF, Horng-Ren Yang123BDE, Kin-Shing Poon36DFDOI: 10.12659/AOT.923502
Ann Transplant 2020; 25:e923502
Abstract
BACKGROUND: ABO-incompatible (ABO-i) living donor liver transplantation (LDLT) is a feasible alternative for donor liver allograft in emergency situations, especially in Asia, where deceased-donor organs remain scarce. The reported outcomes of ABO-i LDLT after optimal desensitization are comparable to those of ABO-compatible LDLT. In this retrospective study, we found improved outcomes after ABO-i LDLT with a low-dose rituximab in combination with double-filtration plasmapheresis (DFPP) and prophylactic antibiotic therapy.
MATERIAL AND METHODS: Between January 2006 and December 2018, a total of 65 recipients underwent ABO-i LDLT surgeries at our center. The study cohort consisted of 50 recipients (Era III) who underwent ABO-i LDLT using the recently updated desensitization protocol, which included rituximab 200 mg intravenous injection once a week prior to LDLT, 4 sessions of DFPP in all patients, and prophylactic antibiotics for 3 months.
RESULTS: The 3-year overall survival rate achieved in ABO-i LDLT patients was 72.7% (66.6% for Era I and 33.3% for Era II patients). In the study population, 11 patients developed complications due to infection. Five of these patients (10%) died due to overwhelming sepsis. Four patients (8%) were diagnosed with multiple strictures and diffusely scattered dilatation of intrahepatic bile ducts on computed tomography, without vascular complications. Three of them had evidence of antibody-mediated rejection (AMR).
CONCLUSIONS: Our experience shows that the ABO-i LDLT protocol of lowered rituximab combined with pre-transplant sessions of plasmapheresis and a quadruple immunosuppressive regimen can be effective in chronic liver failure patients with clinical urgency in the absence of an ABO-compatible donor. Fast-tracking the use of ABO-i LDLT is feasible in patients with an acute liver failure (ALF) and can safely increase the donor liver pool, with an acceptable outcome.
Keywords: Graft Rejection, Living Donors, Plasmapheresis, ABO Blood-Group System, Blood Group Incompatibility, End stage liver disease, Immunologic Factors, Liver Transplantation, rituximab, Survival Rate, Time Factors, Tissue Donors
Background
As the success after liver transplantation for end-stage liver disease continues to increase, the increasing demand for donor liver grafts exceeds the supply. This prompted many transplant centers to expand the living-donor pool by use of ABO-i LDLT, which is a feasible alternative for donor liver allograft in emergency situations, especially in Asia, where deceased-donor organs remain scarce. Despite being regarded as an immunologically privileged organ, outcomes after initial ABO-i liver transplantation (ABO-i LT) surgeries were disappointing, resulting in early liver graft loss, with reported mortality rates as high as 50% of the transplanted recipients within the first year after transplantation [1–3]. The most important causes of the high mortality after ABO-i LT were the high incidence of early graft dysfunction due to acute rejection, vascular complications such as hepatic arterial thrombosis (HAT), and biliary complications. The acute rejections after ABO-i LT were primarily AMR caused by anti-A and anti-B isoagglutinin [4]. In AMR, the preformed recipient isoagglutinins bind to the graft vasculature, resulting in complement activation and migration of neutrophils, which causes vessel damage and consequent activation of the fibrinolytic system, with hemorrhagic necrosis of the graft. Although some studies reported 60% 1-year survival rates, the morbidity rate was high due to the high incidence of complications [5]. The susceptibility to rejection can be adequately explained by blood group antigens that are expressed on the vascular endothelium and in large bile ducts for up to 150 days after transplantation. This caused waning of enthusiasm for incompatible LT, as even the surviving patients had high morbidity rates; thus, the ABO-i LT option was considered only in patients with clinical urgency secondary to deterioration of liver functions and unavailability of a compatible graft [6]. However, in recent years, many Asian studies reported good outcomes after ABO-i LDLT after the introduction of pre-transplant desensitization. Various treatment protocols have been used for iso-titer elimination in ABO-i LDLT patients.
Several therapies were introduced to decrease AMR and improve survival after incompatible transplantation. Desensitization of the recipient with anti-CD20 monoclonal antibody rituximab therapy along with therapeutic plasma exchange treatment and availability of better immunosuppression led to improved ABO-i LT graft survival rates in recent years [7]. Pre-transplant treatment with rituximab, which is an anti-CD20 monoclonal antibody, along with plasmapheresis, have virtually eliminated the risk of graft failure secondary to AMR. In the rituximab era, improved 1-year graft survival rates after ABO-i LT have been reported, ranging from 60% to 100% [8,9]. In their recent retrospective study of 235 ABO-i LDLTs, Song et al. reported a 3-year patient survival rate of 92.3%, with a 7.2% incidence of AMR (n=17) and a mortality rate of 1.27% (n=3) directly related to AMR [10]. Despite these successful reports, ABO-i LDLT remains an uncommon surgery in the Western literature, which is limited to only a few small case series of ABO-i LDLT [11]. In Asia, especially in Taiwan, Japan, and South Korea, the experience in ABO-i LDLT continues to evolve and the use of ABO-i LDLT has continuously developed [12–14]. However, the optimal protocol in ABO-i liver transplantation remains unclear. Here, we present our successful experience with ABO-i LDLT using a reduced dose of rituximab (200 mg single dose) with plasmapheresis and a quadruple immunosuppressive therapy in the post-transplant period. We also discuss the beneficial effects of long-term prophylactic treatment with antibiotics in eliminating early post-transplant septic complications. This study also highlights the safety of fast-tracking ABO-i LDLT within 1 week after de-sensitization therapy.
Material and Methods
PATIENT COHORT:
From September 2002 to December 2018, 1063 liver transplantation surgeries were performed at the China Medical University Hospital, Taiwan. The medical records of adult patients >18 years old who underwent ABO-i LDLT (n=65) were retrospectively analyzed.
We collected and assessed demographic data, medical records, operative details, and postoperative laboratory details, including anti-A and B titers, liver function tests, and B cell count.
Donor selection criteria, evaluation process, and operative technique have been described earlier [15]. The ABO-i LDLT was preferred only in the presence of clinical deterioration of the recipient in the absence of a suitable ABO-compatible living donor. All the recipients received right-lobe liver grafts. For the right liver grafts without a middle hepatic vein, venous tributaries of segment 5 and 8 were reconstructed in a back-table procedure using expanded polytetrafluoroethylene synthetic vascular conduits to form a common outflow channel [16].
All our LDLT patients were approved by the Institutional Review Board and Ethics Committee. The study protocol and results were published previously as an abstract of the 2017 American Transplant Congress.
All the ABO-i recipients received rituximab therapy in the pre-LDLT period and received plasmapheresis as per institutional protocol. For the first 9 recipients (Era I), we used high-dose rituximab (375 mg/m2) and splenectomy, whereas plasmapheresis was performed if anti-A/B agglutinin titers were >1: 64. However, due to the occurrence of adverse events, we modified the protocol for the next 6 patients (Era II, n=6). Plasmapheresis was performed in Era II, but the number of sessions varied. The protocol was later updated to the present protocol with elimination of splenectomy (described later). A total of 50 ABO-i LDLTs were performed using the present desensitization protocol (Era III protocol). Prophylactic antibiotics were given for 3 months after LDLT to all the recipients.
This study mainly focused on the Era III patients (n=50) and the reduced rituximab desensitization protocol. The subgroup of HCC patients in Era III were analyzed further and their outcomes after LDLT were compared with the non-HCC patients.
PROTOCOL OF RITUXIMAB THERAPY, THERAPEUTIC PLASMA EXCHANGE, AND ANTI-A/B TITERS:
All the recipients in this study were hospitalized and received a single intravenous dose of rituximab (200 mg) 4–10 days prior to the LDLT to deplete the B cells. Immunoglobulin M (IgM) and G (IgG) anti-ABO isoagglutinin titers against donor erythrocyte antigens were measured in recipients at the time of hospitalization. The CD-19 B cell count was measured and monitored to check for sufficiency of the dose. In case of a surge in B cells in the post-transplant period, a single dose of rituximab (200 mg) was administered to deplete the B cells further. The target B cell count was 0%.
After 24 hours of rituximab therapy, total plasma exchange was performed by double-filtration plasmapheresis (DFPP) method once a day for 4 consecutive days before LDLT, and was repeated if necessary. The target titers of IgM and IgG isoagglutinin for the donor ABO blood group were less than or equal to 1: 64. In case of failure to achieve the target titers despite several sessions of DFPP, we proceeded with LDLT. The DFPP was performed as a protocol, even in patients with low titers (<1: 16). The DFPP was repeated in the post-transplant period if any increase in ABO antibody titers occurred. After ABO-i LDLT, anti-A/B titers were measured every alternate day starting from the first postoperative day for 1 week and then once a week until patient is discharged. In case of abnormal laboratory findings, the titers were tested and the sessions of DFPP were repeated if deemed necessary. The protocol of the present ABO-i LDLT (Era III) is presented in Figure 1.
POST-LDLT IMMUNOSUPPRESSION AND ANTI-BACTERIAL PROTOCOL:
All the patients in this study received triple immunosuppression consisting of steroids, tacrolimus, and mycophenolate mofetil. Basiliximab (20 mg), a chimeric monoclonal antibody, (Simulect®; Novartis Pharmaceuticals, East Hanover, NJ, USA) was used as an induction agent in all recipients undergoing LDLT. The first dose was administered intravenously during recipient surgery, and the second dose was given on day 4 after LDLT. After February 2012, we started using everolimus as primary immunosuppression along with the tacrolimus. The details of the current immunosuppression regimen have been described earlier [17]. Corticosteroids were withdrawn 15 days after LDLT. Tacrolimus was started from the second postoperative day, with a 1-mg starting dose, and gradually increased to maintain trough levels up to 8–10 ng/ml. MMF 500 mg was given twice a day starting from the second postoperative day. Everolimus, when used, was given 0.25 mg q12 hours and increased to 0.5 mg q12 hours to achieve a target trough level of 3–5 ng/ml.
We administered antibiotics for 3 months after LDLT, according to culture and sensitivity reports, if available, or oral Septran DS (Trimethoprim 80 mg+Sulphamethoxazole 400 mg).
DETECTION OF ACUTE CELLULAR REJECTION AND ANTIBODY-MEDIATED REJECTION:
We did not perform protocol liver biopsy after LDLT for diagnosis of acute rejection episodes. The acute cellular rejection was diagnosed by clinical suspicion and increased levels of liver enzymes (>50 IU/L compared with previous readings) in the absence of technical complications such as HAT. AMR was suspected in the presence of markedly increased liver enzymes and rising anti-A/B agglutinin titers (titers >1: 64). ACR episodes were treated by adjusting the dosage if there was suspicion of AMR, DFPP was repeated, and 1 dose of 150 mg of rituximab was administered. Liver biopsy was always performed when AMR was suspected and in patients with steroid-resistant rejection. Special immunohistochemical analysis was done using a polyclonal antibody against C4d complement. The intensity of C4d staining was quantitatively assessed by the percentage of portal tracts containing distinctly stained stroma and/or endothelium. Biopsies containing >50% stromal-positive portal tracts were considered positive [18].
STATISTICAL ANALYSIS:
Descriptive statistics were computed for categorical variables. These were then examined by independent 2-sample
Results
BASELINE CHARACTERISTIC OF ABO-I PATIENTS:
Out of a total of 1017 LDLTs performed from September 2002 to December 2018, 65 patients received ABO-i LDLT. Initially, 15 patients (Era I, n=9; Era II, n=6) received a different desensitization regimen and 50 patients (Era III) received our updated and modified protocol. Era III ABO-i patients and their outcome were the main focus of the present study.
The mean age of the study cohort (n=50, male: female, 38: 12) was 54±8 years (range, 32–67 years). The primary indication for LDLT was hepatocellular carcinoma (HCC) in 27 (54%) patients, hepatitis B virus (HBV)-related end-stage liver disease (ESLD) in 5 (10%) patients, hepatitis C virus (HCV)-related ESLD in 3 (6%) patients, and 15 patients had other etiologies.
All the patients received a right liver graft in this study. Segment 5 and 8 venous tributaries were reconstructed using expanded polytetrafluoroethylene (ePTFE) synthetic grafts in all patients. The most common donation was from blood group A donor to blood group O recipient (n=20, 40%), followed by blood group B donor to blood group O recipient (n=15, 30%) (Table 1).
PERI-LDLT CD-19 B CELL COUNT, ANTI-A/B ISOAGGLUTININ TITERS, AND PLASMA THERAPEUTIC EXCHANGE TREATMENT:
All patients in the study cohort received a single dose of intravenous rituximab (200 mg) prophylaxis and 4 consecutive sessions of DFPP prior to LDLT. However, in 7 patients, LDLT was done after 1–3 sessions of DFPP within 5 days of rituximab administration due to medical urgency secondary to deteriorating liver functions and worsening encephalopathy. The remaining patients received 4 sessions of DFPP prior to LDLT, even if the titers were below 1: 64. Nine patients required additional DFPP sessions (range, 6–8) due to high anti-A/B titers.
The median initial IgM titer in the ABO-i patients was 1: 64 (range, 1: 2–1: 1024) prior to DFPP and LDLT (Figure 2A, 2B). After plasma exchange treatment, the median IgM titer was 1: 32 (range, 1: 1–1: 128) (Figure 3A, 3B). Only 12 patients (24%) had isoagglutinin titer equal to or more than 1: 64 in the post-LDLT period (5 patients had anti-A/B isoagglutinin titers of 1: 128 after transplantation and 7 patients had titers of 1: 64). No patients in this series had rebound rise in anti-A/B titers in the postoperative period compared to their pre-LDLT levels. However, 2 patients required more than 2 sessions of DFPP in the post-LDLT period due to initial rise in liver enzymes that did not respond to increased immunosuppressive doses. No LDLT operations were aborted if the titers failed to decrease after desensitization therapy.
The CD-19 B cell count was measured and followed in all patients before rituximab injection, 1 day prior to LDLT, and in the postoperative period. The mean CD-19 B cell count before rituximab administration was 16±10.8% (range, 2.1–54.2%). After rituximab and DFPP sessions, the mean CD-19 B cell count was 0.5±1.0% (range, 0–6.0). In the first week after LDLT, the mean CD-19 B cell count was 0.7±1.1% (range, 0–6.5%) (Figure 4A, 4B).
INFECTION-RELATED COMPLICATIONS, ACUTE CELLULAR REJECTION EPISODES, AND SURVIVAL OUTCOMES:
Fatal infection-related complications were more common in Era I and II patients. In Era II patients (n=6), 5 developed infectious complications and died of overwhelming sepsis, and all of these patients had undergone splenectomy. In the present study population (n=50), 11 patients developed infection-related complications. Two patients were diagnosed with biliary tract sepsis, 1 patient had pneumonia due to Pneumocystis carinii infection, and 2 patients had multidrug-resistant Acinetobacter-related sepsis. Cytomegalovirus infection was detected in 2 patients and was successfully treated and 2 patients developed herpes virus reactivation. Two patients had respiratory tract infection in the postoperative period and were successfully treated with broad-spectrum antibiotics. One patient had acute respiratory tract distress syndrome (ARDS) secondary to rituximab; no infectious source was found, and the patient required extra-corporal membrane oxygenation (ECMO) in the postoperative period. The patient recovered from ARDS; however, his hospital stay was prolonged for 3 months after LDLT. The details of this case have been reported earlier [18]. Five of these patients (10%) died due to overwhelming sepsis (Table 2).
Seven patients (14%) developed acute cellular rejection episodes that were diagnosed when the aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were elevated to >50 IU/L compared to the previous day or twice the normal level in the absence of infection and/or vascular complications of the liver allograft. None of the patients in this series developed steroid-resistant acute rejection. Two patients received steroid pulse therapy and the other 5 patients were managed by increasing the immunosuppressive dose.
DIFFUSE INTRAHEPATIC BILIARY STRICTURE PATIENTS (DIHBS), AMR, AND MANAGEMENT:
Four patients (8%) were diagnosed with multiple strictures and diffusely scattered dilatation of intrahepatic bile ducts on computed tomography without HAT. The time of detection varied from 2 to 4 months after LDLT. The details of laboratory results of these patients are presented in Table 3. Liver biopsy was done in all 4 patients to establish the diagnosis. Two patients had biopsy-proven AMR. The first 3 patients underwent successful re-transplantation by deceased-donor liver transplantation and continues to be under regular follow-up, with stable graft functions, and the fourth patient with DIHBS died due to sepsis.
ANASTOMOTIC BILIARY STRICTURES AND VASCULAR COMPLICATIONS:
Fifteen patients (30%) developed anastomotic biliary strictures that required endoscopic interventions and stent placements. None of these patients had cholangitis episodes. Two patients (2%) had HAT that needed re-exploration and revision of hepatic arterial anastomosis. These patients recovered uneventfully thereafter without further complications.
ABO-I LDLT IN THE HCC SUBGROUP OF PATIENTS AND OUTCOMES:
In this study, the subgroup of HCC consisted of 27 patients; 16 patients (32%) were within UCSF criteria (subgroup A) and 11 patients (22%) had advanced HCC beyond the UCSF criteria (subgroup B). The average AFP levels were 33 ng/ml (range, 2–137) for subgroup A. Four patients from subgroup B (n=11) had AFP >2000 ng/ml (range, 2805–54000) and the remaining 7 patients had AFP levels <200 ng/ml. Three patients in this subgroup had prior hepatectomy and underwent LDLT due to recurrence. The explant pathology vascular invasion results are shown in Table 4. In the subgroup A cohort, only 1 patient had microvascular invasion on explant pathology, whereas 2 patients in subgroup B had macrovascular invasion detected on explant pathology. Four patients in the HCC cohort developed DIHBS. Liver biopsies in these patients did not show conclusive evidence of AMR. One patient in subgroup A and 4 patients in subgroup B died due to HCC recurrence.
Overall, the 3-year survival in the HCC cohort (n=27) was 56%. Nevertheless, for the patients in the UCSF group (n=16), the 1-year and 3-year survival rates achieved in subgroup A patients were 100% and 90.48%, respectively. The cumulative 3-year survival rate for subgroup B patients was 63.6%. Four patients in subgroup B (beyond UCSF) died (2 due to HCC recurrence and 2 due to sepsis).
OVERALL SURVIVAL:
The 3-year overall survival rate in ABO-i LDLT patients was 72.7% (66.6% for Era I and 33.3% for Era II patients) (Figure 5). The survival rate of ABO-compatible LDLT patients (n=426) in the same time period was 78.3% (Figure 6). In the study cohort, patients were divided into HCC and non-HCC groups. The 3-year survival rate in non-HCC patients (n=23) was 83.6%. For HCC patients within UCSF criteria (n=16), the 3-year survival rate was 90.5% compared to 81.3% in ABO-compatible HCC patients within UCSF criteria (n=107) during the same study period (Figure 7). Three patients had acute liver failure with hepatic coma. After 1.5 years of follow-up, the survival rate was 66.6%
Discussion
This retrospective study shows the safety and feasibility of fast-tracking ABO-i LDLT within 7 days after rituximab injection, with a long-term outcome comparable to ABO-compatible liver transplant surgery (3-year survival 72.7%
The indications for ABO-i LDLT can be extended to patients with ALF by reducing the desensitization period after rituximab injection. However, a pre-LDLT desensitization period of 2 weeks is considered necessary for successful outcomes after ABO-i LDLT. Therefore, ALF patients who require urgent LDLT are usually regarded as ineligible for ABO-i LDLT. However, the ALF patients can be fast-tracked by shortening the preparatory period for ABO-i LDLT with a reduced rituximab dose and quick sessions of plasmapheresis. The only concern in fast-tracking the ABO-i LDLT is the therapeutic efficacy of rituximab injection in depleting the CD20+ cells if plasmapheresis sessions are performed within 1–3 days after its administration [21]. Recent studies showed markedly increased rituximab clearance when plasmapheresis was performed within 48 hours, although its impact on therapeutic effects were not analyzed [22,23]. The rapid effects of rituximab in reduction of CD 20+ within 24–36 hours after infusion are not altered by regularly scheduled plasmapheresis [24]. Egawa et al., in a multicenter study, found no association between timing of rituximab injection and the incidence of AMR [25]. Therefore, we can cautiously consider ABO-i LDLT as an option in patients with ALF with an earlier start of the first session of plasmapheresis after infusion of rituximab. In this series, 7 patients underwent LDLT within 4 days of rituximab administration. Three patients had ALF and 4 chronic cirrhotic patients had rapid clinical deterioration due to acute decompensation of hepatic reserve. Two patients died (1 ALF and 1 ACLD) within 3 months of LDLT. There was no incidence of AMR in this subgroup. The cause of death in both patients in this subgroup was sepsis.
The role of LT for patients with non-resectable HCC is well established, as it can achieve complete tumor excision and removal of the carcinogenic liver. However, tumor recurrence remains the main cause of death in this subset of patients due to the patient’s immunosuppressed state. The population of HCC patients that require LT continues to increase in Asia. The need for extended-criteria and marginal-donor grafts has been the topic of debate for patients with advanced and/or unresectable HCC. Despite the potential for living-donor pool expansion with ABO-i living donors, the possible effects of rituximab and plasmapheresis on tumor recurrence remain a concern [26]. Rituximab injection significantly depletes tumor-infiltrating B cells, which enhances tumor growth and reduces local T cell activation [27]. In this retrospective analysis, the survival of HCC patients within UCSF criteria who underwent ABO-i was comparable to that of the similar HCC population that underwent ABO-compatible LDLT (3-year survival of 90.5%
In the present era, survival outcomes after ABO-i LT have improved, but concerns about AMR persist. The diagnosis of AMR is based on a combination of parameters that include clinical, serological, and histological findings. The published literature suggests that DIHBS occurring several months after ABO-i LDLT can be a clinical manifestation of AMR [10,30]. The higher incidence of biliary strictures in ABO-i LDLT recipients is considered to be due to direct immunological mechanisms such as bile duct epithelium expressing A and B blood group antigens [31]. In the present study, no patients developed hepatic necrosis. DIHBS, a manifestation of AMR, is not always fatal and has a wide disease spectrum, including silent rise of liver enzymes without clinical symptoms and cholangitis leading to sepsis and graft failure. Four patients who developed DIHBS in this series underwent liver biopsy. Two patients had biopsy-proven AMR that developed at 2 and 3 months after LDLT, respectively, but there was no conclusive histopathological evidence of AMR in the remaining 2 patients. DIHBS cannot be resolved with conventional endoscopic or percutaneous treatment modalities for biliary strictures. Patients with multiple biliary strictures require frequent hospital admissions for the recurrent cholangitis, which significantly affects quality of life. Hence, re-transplantation remains the only proven effective treatment in such patients. Three patients who developed DHIBS in our study underwent successful re-transplantation, while 1 patient with DIHBS died due to biliary sepsis.
A higher isoagglutinin titer in the postoperative period is considered to be a risk factor for the development of AMR, although the role of ABO antibody titer is unclear [6,32]. Song et al. showed antibody rebound in 20% of patients, but there was no correlation between the incidence of AMR and increased agglutinin titers, and the rate of AMR-free survival was 96% in the patient population with antibody titers >1: 64 [10]. The correlations among the incidence of AMR, blood type, and agglutinin titers remain controversial [33,34]. However, low agglutinin titers should always be the aim. Higher pre-transplant titers can be effectively lowered by plasmapheresis, which can be repeated if there is any rebound increase in titers in the post-LT period. The rituximab prophylaxis is very effective in suppressing the B lymphocyte population, but its effects on antibody production by plasma cells that do not express CD20 is minimal. In our study, 6 patients (12%) had rebound increase in antibody titers in the post-LDLT period. Two patients with DIHBS had anti-A/B agglutinin titers more than 1: 512 and 1 of these patients showed histopathological evidence of AMR on liver biopsy. Bortezomib, a recently introduced proteasome inhibitor, selectively induces apoptosis of plasma cells and thus decreases rebound antibody production [35].
This study highlights several important points. First, the rituximab dose can safely be reduced. Second, the fast-tracking of ABO-i LDLT is feasible, with successful long-term outcomes comparable to ABO-compatible LDLT. Third, DIHBS incidence is higher in patients with ABO-i LDLT, and re-transplantation is the only lifesaving procedure for this subgroup of patients. The fast-tracking of ABO-i LDLT after rituximab therapy and rapid sessions of plasmapheresis is a plausible approach. Earlier studies have proven the rapid effects of rituximab on B cells, which are eliminated within 48–72 hours of therapy, and a single dose is sufficient for sustained suppression of B cells for several months in the post-transplant period [36]. In this retrospective analysis, we reduced the rituximab dose to 200 mg without increased incidence of graft failure or AMR.
Conclusions
Our ABO-i LDLT protocol of lowered rituximab combined with pre-transplant sessions of plasmapheresis and quadruple immunosuppressive regimen (basiliximab, tacrolimus, steroids, and mycophenolate mofetil) can be effective in chronic liver failure patients with clinical urgency in the absence of an ABO-compatible donor. The rebound antibody titer, although an alarming event in the post-LDLT period, is not correlated with an increased incidence of AMR. ABO-I LDLT is an effective and safe transplant option for HCC patients in the absence of a compatible donor. The rituximab prophylaxis and plasmapheresis do not increase the risk of HCC recurrence after LDLT. The results after ABO-i LDLT in patients with HCC beyond the UCSF criteria are also promising. The present results show that fast-tracking ABO-i LDLT is feasible and can increase the donor liver pool, but further studies are needed to confirm this.
Figures
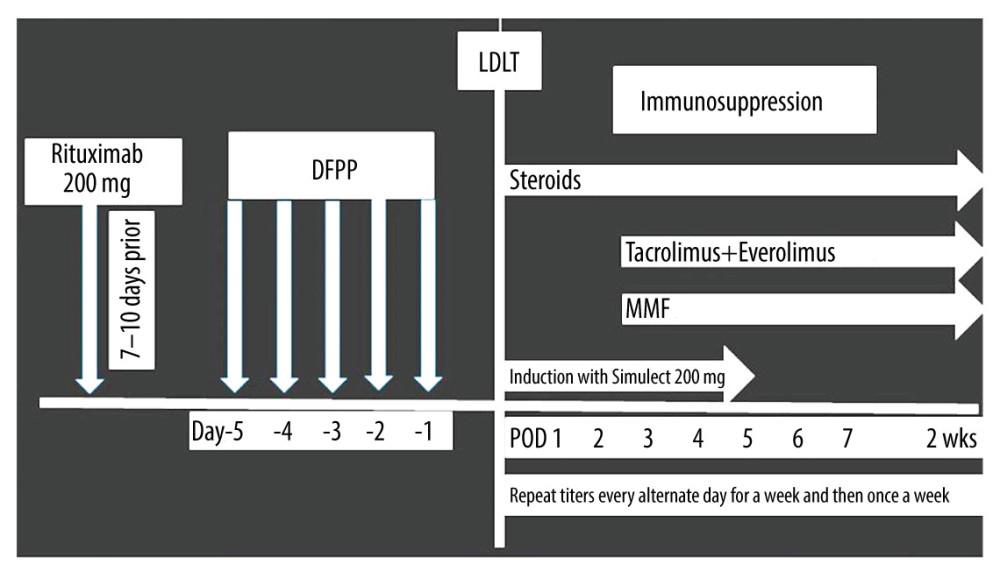 Figure 1. Institution of the ABO-i LDLT protocol for the timing of the rituximab injection and postoperative follow-up.
Figure 1. Institution of the ABO-i LDLT protocol for the timing of the rituximab injection and postoperative follow-up. 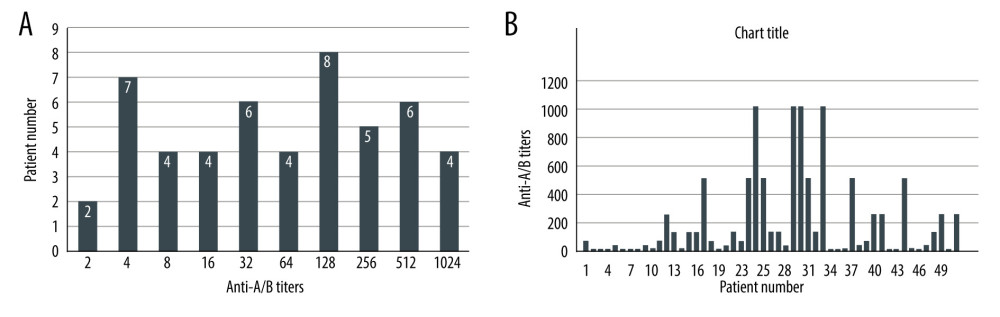 Figure 2. (A, B) Pre-rituximab titers in Era III patient population.
Figure 2. (A, B) Pre-rituximab titers in Era III patient population. 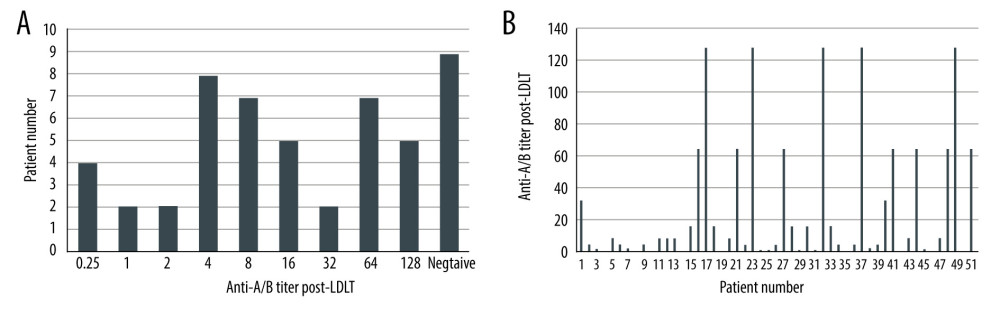 Figure 3. (A, B) Post-LDLT anti-A/B isoagglutinin titers.
Figure 3. (A, B) Post-LDLT anti-A/B isoagglutinin titers. 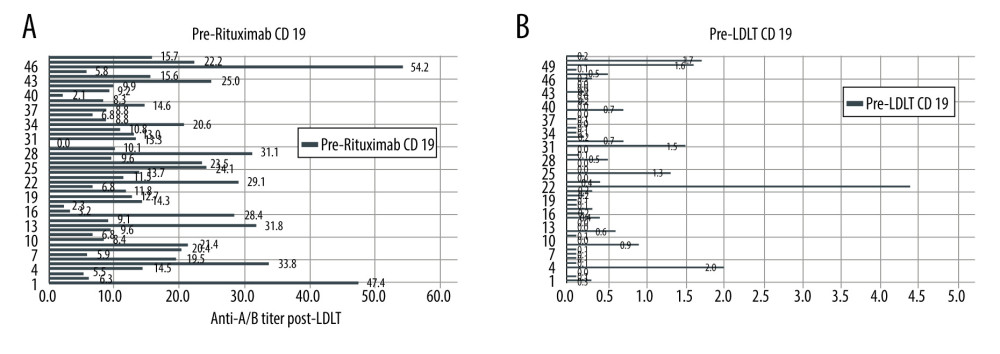 Figure 4. (A) Graph showing pre-rituximab CD-19 cell count. (B) Graph showing pre-LDLT CD-19 cell count.
Figure 4. (A) Graph showing pre-rituximab CD-19 cell count. (B) Graph showing pre-LDLT CD-19 cell count. 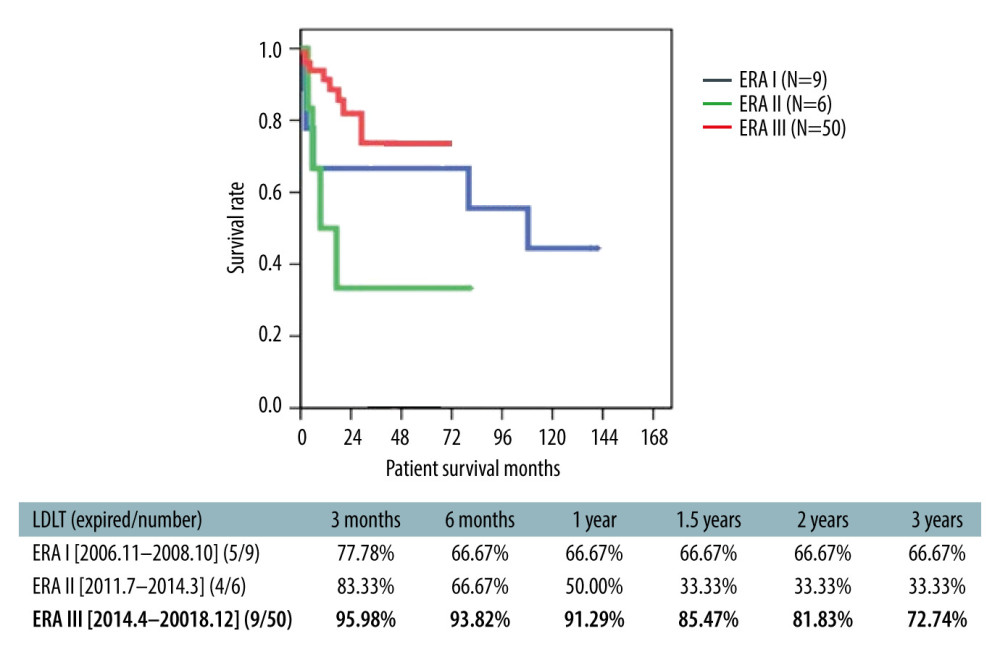 Figure 5. Overall survival of ABO-i LDLT patients.
Figure 5. Overall survival of ABO-i LDLT patients. 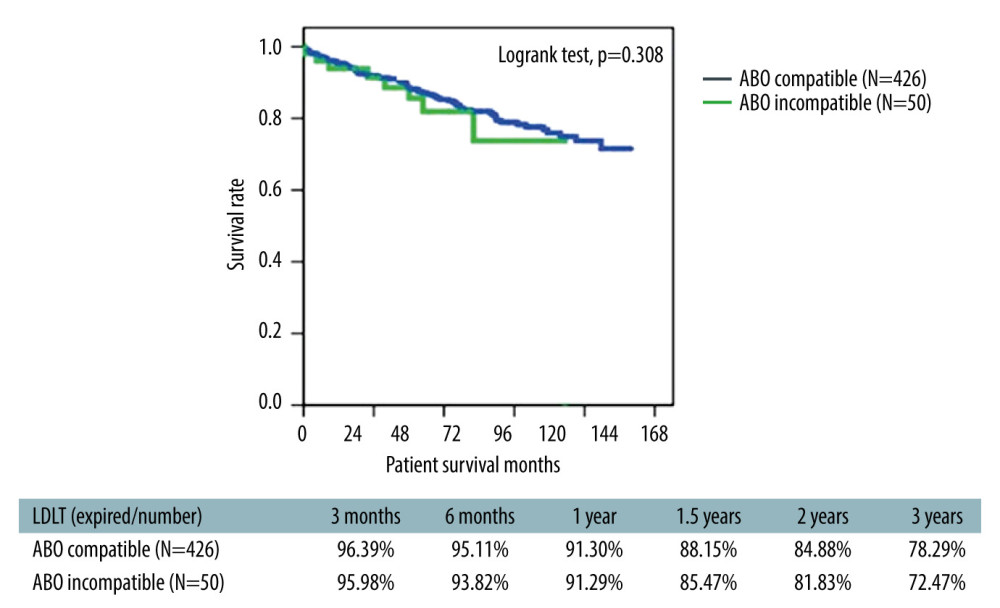 Figure 6. Survival analysis comparing ABO-i LDLT and ABO-compatible LDLT patients.
Figure 6. Survival analysis comparing ABO-i LDLT and ABO-compatible LDLT patients. 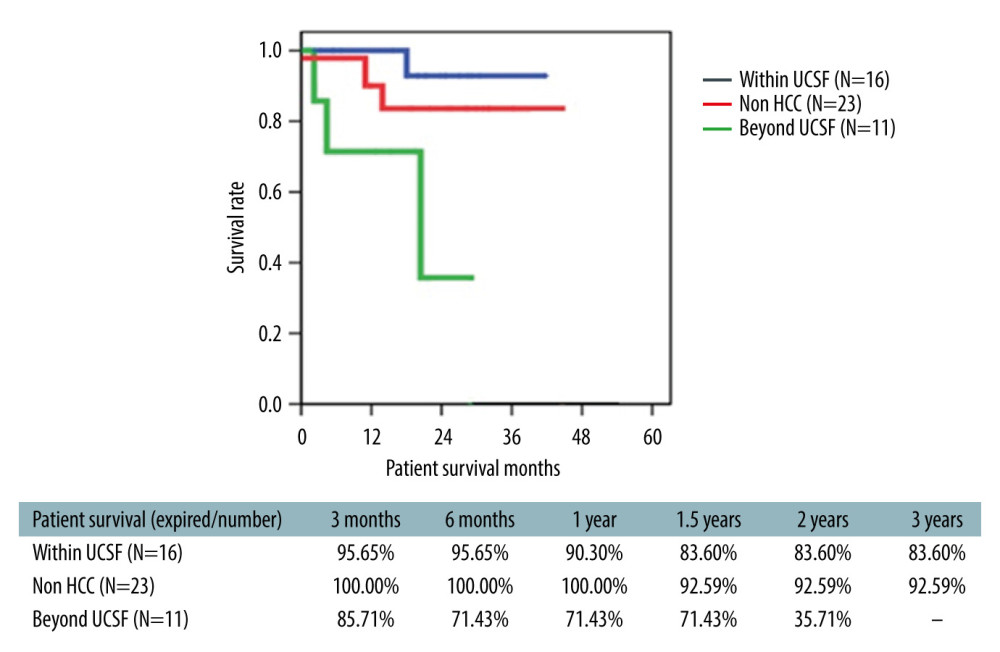 Figure 7. Survival of ABO-i LDLT recipient with and without HCC.
Figure 7. Survival of ABO-i LDLT recipient with and without HCC. References
1. Gugenheim J, Samuel D, Reynes M, Liver transplantation across ABO blood group barriers: Lancet, 1990; 336; 519-23
2. Lo C, Shaked A, Busuttil RW, Risk factors for liver transplantation across the ABO barrier: Transplantation, 1994; 58; 543-47
3. Sanchez-Urdazpal L, Batts KP, Gores GJ, Increased bile duct complications in liver transplantation across the ABO barrier: Ann Surg, 1993; 218; 152-58
4. Stewart ZA, Locke JE, Montgomery RA, ABO-incompatible deceased donor liver transplantation in the United States: A national registry analysis: Liver Transpl, 2009; 15; 883-93
5. Farges O, Kalil AN, Samuel D, The use of ABO-incompatible grafts in liver transplantation: A life-saving procedure in highly selected patients: Transplantation, 1995; 59; 1124-33
6. Raut V, Uemoto S, Management of ABO-incompatible living-donor liver transplantation: Past and present trends: Surg Today, 2011; 41; 317-22
7. Hanto DW, Fecteau AH, Alonso MH, ABO-incompatible liver transplantation with no immunological graft losses using total plasma exchange, splenectomy, and quadruple immunosuppression: Evidence for accommodation: Liver Transpl, 2003; 9; 22-30
8. Yamada Y, Hoshino K, Morikawa Y, Successful liver transplantation across the ABO incompatibility barrier in 6 cases of biliary atresia: J Pediatr Surg, 2006; 41; 1976-79
9. Heffron T, Welch D, Pillen T, Successful ABO-incompatible pediatric liver transplantation utilizing standard immunosuppression with selective postoperative plasmapheresis: Liver Transpl, 2006; 12; 972-78
10. Song GW, Lee SG, Hwang S, ABO-incompatible adult living donor liver transplantation under the desensitization protocol with rituximab: Am J Transplant, 2016; 16; 157-70
11. Rummler S, Bauschke A, Baerthel E, ABO-incompatible living donor liver transplantation in focus of antibody rebound: Transfus Med Hemother, 2017; 44; 46-51
12. Lee CF, Cheng CH, Wang YC, Adult living donor liver transplantation across ABO-incompatibility: Medicine (Baltimore), 2015; 94; e1796
13. Raut V, Mori A, Kaido T, Splenectomy does not offer immunological benefits in ABO-incompatible liver transplantation with a preoperative rituximab: Transplantation, 2012; 93; 99-105
14. Song GW, Lee SG, Hwang S, Successful experiences of ABO-incompatible adult living donor liver transplantation in a single institute: No immunological failure in 10 consecutive cases: Transplant Proc, 2013; 45; 272-75
15. Jeng LB, Thorat A, Yang HR, “Rooftop and Skeletonization Technique” of hepatic transection to include or exclude the middle hepatic vein during donor hepatectomy in living donor liver transplantation: Solving the middle hepatic vein controversy – experience in 397 sequential live donors: Med Sci Tech, 2016; 57; 6-15
16. Thorat A, Jeng LB, Yang HR, Outflow reconstruction for right liver allograft with multiple hepatic veins: “V plasty” of hepatic veins to form a common outflow channel versus 2 or more hepatic vein-to-inferior vena cava anastomoses in limited retrohepatic space: Liver Transpl, 2016; 22; 192-200
17. Jeng LB, Thorat A, Hsieh YW, Experience of using everolimus in the early stage of living donor liver transplantation: Transpl Proc, 2014; 46; 744-48
18. Haga H, Egawa H, Fujimoto Y, Acute humoral rejection and C4d immunostaining in ABO blood type-incompatible liver transplantation: Liver Transpl, 2006; 12; 457-64
19. Li Ping-Chun, Thorat Ashok, Hsu Shih-Chao, Successful management of a rare complication and a review of the literature: Ann Transplant, 2017; 22; 463-67
20. Thorat A, Yang HR, Jeng LB, Combined endoscopic therapy and percutaneous approach in patients with biliary strictures after adult right-lobe living-donor liver transplantation: Surg Gastroenterol Oncol, 2019; 24; 137-46
21. Winters JL, Plasma exchange: Concepts, mechanisms, and an overview of the American Society of Apheresis guidelines: Hematology Am Soc Hematol Educ Program, 2012; 2012; 7-12
22. Genberg H, Hansson A, Wernerson A, Pharmacodynamics of rituximab in kidney allotransplantation: Am J Transplant, 2006; 6; 2418-28
23. Puisset F, White-Koning M, Kamar N, Population pharmacokinetics of rituximab with or without plasmapheresis in kidney patients with antibody-mediated disease: Br J Clin Pharmacol, 2013; 76; 734-40
24. Lee SD, Kim SH, Kong SY, Kinetics of B, T, NK lymphocytes and isoagglutinin titers in ABO incompatible living donor liver transplantation using rituximab and basiliximab: Transpl Immunol, 2015; 32; 29-34
25. Egawa H, Teramukai S, Haga H, Impact of rituximab desensitization on blood-type-incompatible adult living donor liver transplantation: A Japanese multicenter study: Am J Transplant, 2014; 14; 102-14
26. Kim JM, Kwon D, Joe JW, ABO-incompatible living donor liver transplantation with rituximab and total plasma exchange does not increase hepatocellular carcinoma recurrence: Transplantation, 2018; 102; 1695-701
27. Garnelo M, Tan A, Her Z, Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma: Gut, 2017; 66; 342-51
28. Lee SD, Kim SH, Kong SY, Kinetics of B, T, NK lymphocytes and isoagglutinin titers in ABO incompatible living donor liver transplantation using rituximab and basiliximab: Transplant Immunol, 2015; 32; 29-34
29. Nydegger UE, Tevaearai H, Berdat P, Histo-blood group antigens as allo- and autoantigens: Ann NY Acad Sci, 2005; 1050; 40-51
30. Egawa H, Ohdan H, Haga H, Current status of liver transplantation across ABO blood-type barrier: J Hepatobiliary Pancreat Surg, 2008; 15; 131-38
31. Sanchez-Urdazpal L, Batts KP, Gores GJ, Increased bile duct complications in liver transplantation across the ABO barrier: Ann Surg, 1993; 218; 152-58
32. Song GW, Lee SG, Hwang S, Biliary stricture is the only concern in ABO-incompatible adult living donor liver transplantation in the rituximab era: J Hepatol, 2014; 61; 575-82
33. Skogsberg U, Breimer ME, Friman S, Adult ABO-incompatible liver transplantation, using A and B donors: Xenotransplantation, 2006; 13; 154-59
34. Egawa H, Teramukai S, Haga H, Present status of ABO-incompatible living donor liver transplantation in Japan: Hepatology, 2008; 47; 143-52
35. Perry DK, Burns JM, Pollinger HS, Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production: Am J Transplant, 2009; 9; 201-9
36. Uchiyama H, Mano Y, Taketomi A, Kinetics of anti-blood type isoagglutinin titers and B lymphocytes in ABO-incompatible living donor liver transplantation with rituximab and plasma exchange: Transplantation, 2011; 92; 1134-39
Figures
 Figure 1. Institution of the ABO-i LDLT protocol for the timing of the rituximab injection and postoperative follow-up.
Figure 1. Institution of the ABO-i LDLT protocol for the timing of the rituximab injection and postoperative follow-up. Figure 2. (A, B) Pre-rituximab titers in Era III patient population.
Figure 2. (A, B) Pre-rituximab titers in Era III patient population. Figure 3. (A, B) Post-LDLT anti-A/B isoagglutinin titers.
Figure 3. (A, B) Post-LDLT anti-A/B isoagglutinin titers. Figure 4. (A) Graph showing pre-rituximab CD-19 cell count. (B) Graph showing pre-LDLT CD-19 cell count.
Figure 4. (A) Graph showing pre-rituximab CD-19 cell count. (B) Graph showing pre-LDLT CD-19 cell count. Figure 5. Overall survival of ABO-i LDLT patients.
Figure 5. Overall survival of ABO-i LDLT patients. Figure 6. Survival analysis comparing ABO-i LDLT and ABO-compatible LDLT patients.
Figure 6. Survival analysis comparing ABO-i LDLT and ABO-compatible LDLT patients. Figure 7. Survival of ABO-i LDLT recipient with and without HCC.
Figure 7. Survival of ABO-i LDLT recipient with and without HCC. Tables
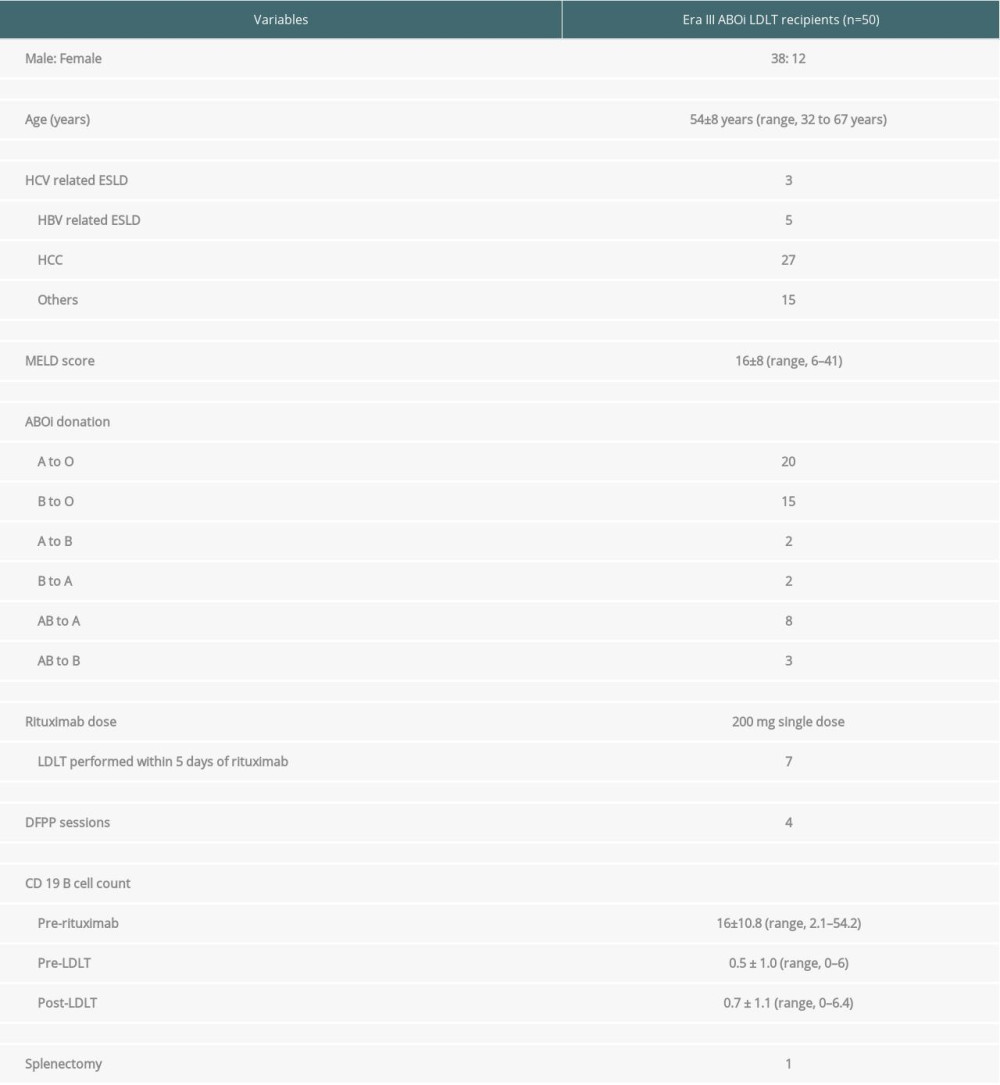 Table 1. General characteristics of study cohort.
Table 1. General characteristics of study cohort.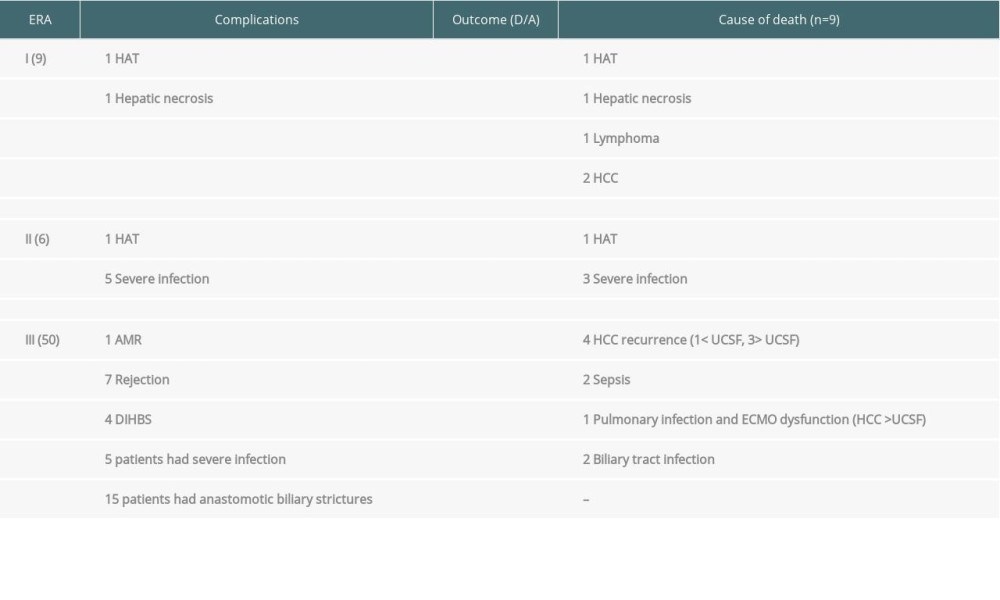 Table 2. Overview of complications in ABOi LDLT study cohort.
Table 2. Overview of complications in ABOi LDLT study cohort.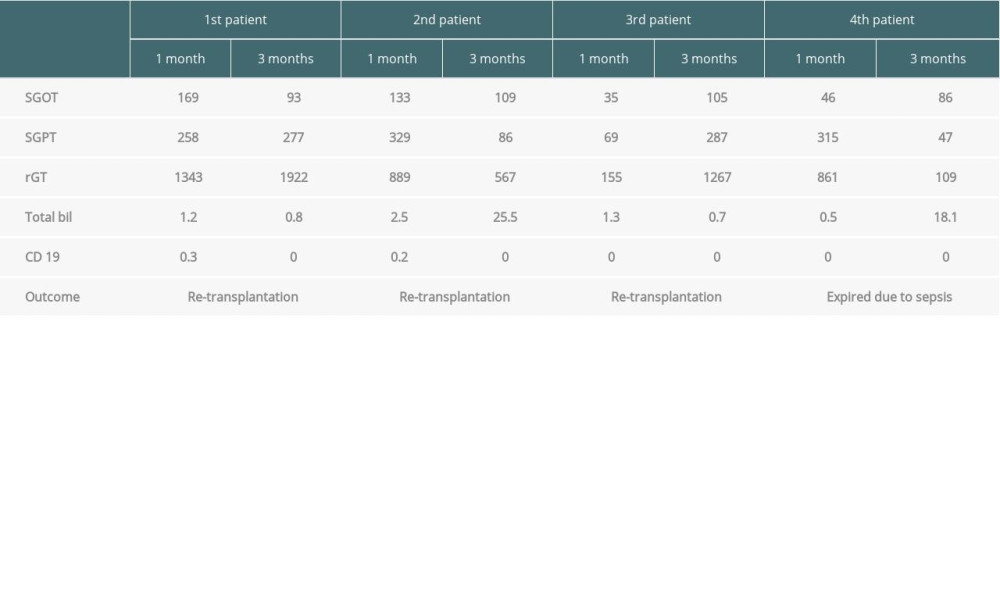 Table 3. DIHBS patient data.
Table 3. DIHBS patient data.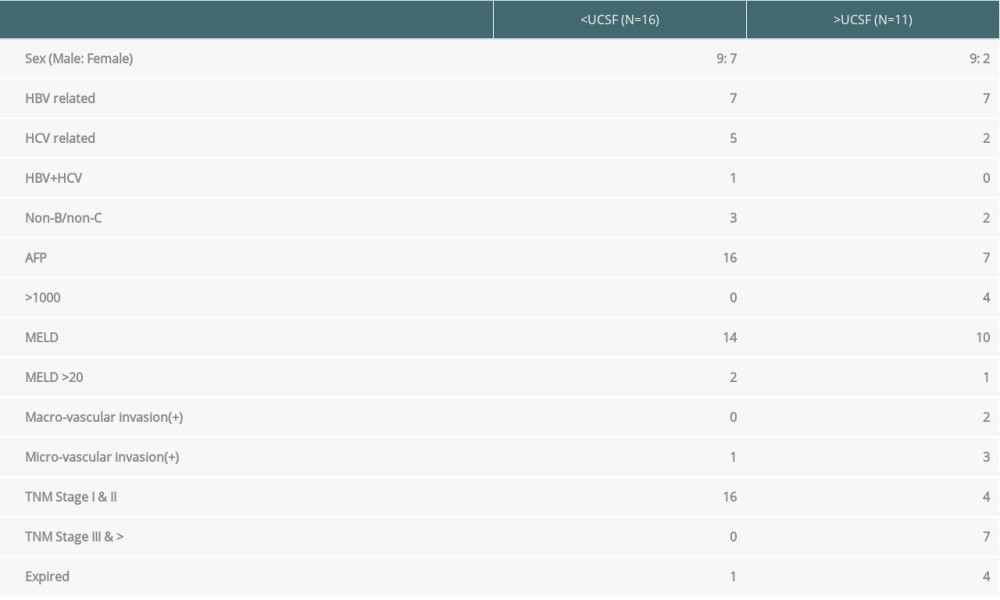 Table 4. Characteristics of HCC patients.
Table 4. Characteristics of HCC patients. Table 1. General characteristics of study cohort.
Table 1. General characteristics of study cohort. Table 2. Overview of complications in ABOi LDLT study cohort.
Table 2. Overview of complications in ABOi LDLT study cohort. Table 3. DIHBS patient data.
Table 3. DIHBS patient data. Table 4. Characteristics of HCC patients.
Table 4. Characteristics of HCC patients. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








