19 June 2020: Original Paper
Safety and Efficacy of Right Retroperitoneal Laparoscopic Live Donor Nephrectomy: A Retrospective Single-Center Study
Yaowen Fu1ABCEF, Yu Hu2ABCEF, Weigang Wang1ABCF, Baoshan Gao1ABCE, Gang Wang1ABCE, Xin Lian1ABC, Honglan Zhou1ABEG*, Yuantao Wang1AEGDOI: 10.12659/AOT.919284
Ann Transplant 2020; 25:e919284
Abstract
BACKGROUND: The aim of this study was to investigate the efficacy and safety of right retroperitoneal laparoscopic live donor nephrectomy (LDN) in 81 cases of living-related renal transplant.
MATERIAL AND METHODS: We retrospectively reviewed all living-related donors who underwent right retroperitoneoscopic living donor nephrectomy between June 2010 and December 2017 at the First Hospital of Jilin University and their corresponding recipients. Demographic and clinical data were collected from the hospital’s electronic clinical data system. Data on preoperative renal retention parameters, operative time, and donor kidney warm ischemia time, the trimmed length of the renal artery and vein of donor kidney, and the time to extubation were recorded. Complications in both donors and recipients were recorded.
RESULTS: We included 81 donors who underwent successful right-sided retroperitoneoscopic LDN, with 31 males and 50 females and a mean age of 47.1 years (range 21–63 years). There was no intraoperative conversion to open donor nephrectomy. The mean operative time was 120.68±29.8 min. The mean warm ischemic time was 49.26±3.86 s. The estimate blood loss was 54.32 mL (range 50–400 mL). The median length of hospital stay was 7 days (range 4–13 days). There was neither intraoperative complication such as hemorrhage or lymph fistula nor kidney graft injury. There was no graft renal vein thrombosis and ureteral stricture or other complications. No graft rejection occurred.
CONCLUSIONS: Right retroperitoneal laparoscopic live donor nephrectomy is safe and effective for renal transplant in living-related renal transplant by laparoscopic excision and extraction of the right kidney with vena cava flap.
Keywords: Kidney Transplantation, Renal Artery, Renal Veins, Kidney, Laparoscopy, Living Donors, Nephrectomy, Tissue and Organ Harvesting, warm ischemia, young adult
Background
Traditional surgical techniques of open donor nephrectomy (ODN) have transitioned over the years to minimally invasive surgical techniques [1]. Compared to ODN, laparoscopic live donor nephrectomy (LDN) is less invasive and has faster recovery, and has become the preferred approach for nephrectomy [2]. Because the right renal vein is shorter, excision and transplantation of the right kidney are challenging, and given a choice, most surgeons prefer left nephrectomy. However, if the left kidney has multiple vessels, the right kidney becomes the only choice available [3].
Right laparoscopic LDN is routinely performed in large-volume centers, but in smaller centers it is less frequently performed due to the technical challenge of the surgical procedure and due to fear of poor outcomes, despite available evidence demonstrating safety and feasibility of the procedure [4,5]. Data are scant on the efficacy and safety of right retroperitoneoscopic nephrectomy in Chinese liver donors, as live kidney transplant is uncommon in China. Ma et al. studied 19 right
Material and Methods
DONORS:
Eligible donors were informed of the risk of the surgical procedures and all provided written informed consent. Organ donation and the study protocol were approved by the local ethics review committee at the authors’ affiliated hospital and by the provincial health ministry of Jilin, China.
All donors who underwent retroperitoneoscopic living donor nephrectomy between June 2010 and December 2017 at the First Hospital of Jilin University and their corresponding recipients were retrospectively reviewed. Donors who were healthy on preoperative workup were included. Donors whose unilateral glomerular filtration rate (GFR) was >40 mL/min and total GFR >80 mL/min were eligible. Persons who had a history of hypertension, cardiac diseases, pulmonary tuberculosis, diabetes mellitus, or chronic hepatic or renal diseases were ineligible.
DONOR EVALUATION:
All donors underwent a preoperative workup according to the Amsterdam Forum guidelines [7]. Renal function was assessed by conventional renal scintigraphy. The anatomy of the renal parenchyma and the renal arteries and veins were imaged by ultrasonography, magnetic resonance angiography, and/or computed tomography (CT)-angiography. The branches of donor renal vessels and malformations of the urinary system were examined by 3-dimensional (3D) CT reconstruction of the urinary system. The selection of the side of LDN was based on leaving the best kidney with the donor [8]. When bilateral GFR differed by less than 10% and if no unilateral anatomical abnormalities were present, anatomical considerations guided surgical decision-making, and if there were no differences between the 2 kidneys, left-sided retroperitoneoscopic LDN was given preference. Right-sided retroperitoneoscopic LDN was given preference if there was an accessory renal artery arising from the abdominal aorta, if there was early branching of the left renal artery (defined as branches arising within 15 mm from the origin of the main renal artery ostium), and if the right GFR was lower than the left GFR by more than 10%.
SURGICAL TECHNIQUE:
The surgery was performed by 3 surgeons with more than 10 years of experience in laparoscopic surgery. All surgical subjects or their legal surrogates provided written informed consent for the surgery.
Under general anesthesia, the patient was placed in the left flank position with the head lowered 15 degrees and the feet 30 degrees, and the lumbar region elevated. Three ports were established. The distance was 6–8 cm between the 2 subcostal operating ports, facilitating the connection of the 2 ports during kidney extraction and controlling incision length (Figure 1). The intraabdominal pressure was set to 1.330–1.596 kPa (10–12 mmHg) to avoid any effect on renal perfusion. Dissection was performed along the surface of the kidney, and the ventral and dorsal aspects and the upper pole of the kidney were dissected. Then, the lower pole of the kidney and the ureter were fully dissociated. The renal artery was dissected distally posterolateral to the inferior vena cava near the origin of the abdominal aorta to assure adequate length of the artery. The renal vein and the inferior vena cava were carefully dissected at their confluence, and fibrofatty tissues 1–2 cm at the caudal and cranial end of the inferior vena cava were removed to facilitate clamping and suturing during renal vein extraction. Furosemide (40 mg) was given intravenously before kidney removal. Urine output was maintained at a brisk pace using aggressive intravenous hydration. The ureter was carefully dissected to assure adequate perfusion. Pneumoperitoneum was released before the renal pedicle was cut and the kidney retrieved. A transverse incision connecting the 2 operating ports was made to allow the entry of half of a curled hand. The incision was retracted cranially and ventrally by 2 large S hookers. The peritoneum was not violated, but it, along with the gastrointestinal tract, was gently pushed away to expose the kidney and the renal pedicle. A surgical space identical to that under the pneumoperitoneum was established. Under direct laparoscope monitoring, the renal artery was clipped proximally with 2 Hem-o-lok clips and cut. The renal vein was clamped at the confluence of the renal vein at the inferior vena cava and the inferior vena cava and cut with a laparoscopic scissor so that the right renal vein contained a partial vena cava wall (Figure 2). The donor kidney was rapidly retrieved along the long axis of the kidney. The incision on the lateral wall of the inferior vena cava was continuously sutured using 4-proline and examined for active bleeding or lymphatic oozing before the wound was closed in layers. The surgical steps are shown in Figure 3.
DATA COLLECTION:
Demographic and clinical data were collected from the hospital’s electronic clinical data system. Data on preoperative renal retention parameters, body mass index (BMI), blood group, and HLA status of both donors and recipients were obtained. Operative time was defined as the interval from the moment when pneumoperitoneum was established to the time when anastomosis of the vena cava incision was completed. Donor kidney warm ischemia time was defined as the interval from clamping of the renal artery to the start of hypothermic perfusion. The trimmed length of the renal artery and vein of donor kidney and the time to extubation were recorded. The length of hospital stay was calculated from the date of surgery to the date when the patient was discharged from the hospital. Delayed graft function was defined as the use of dialysis in the first postoperative week [9]. Recipients were followed up at our surgical outpatient clinic at 3 months after surgery and after transplant renal function was noted. Complications in donors and recipients were recorded.
STATISTICAL ANALYSIS:
Data are expressed in mean±standard deviation (χ±s) and were analyzed using SPSS 21 (SPSS, Inc., Chicago IL, USA).
Results
DONOR CHARACTERISTICS:
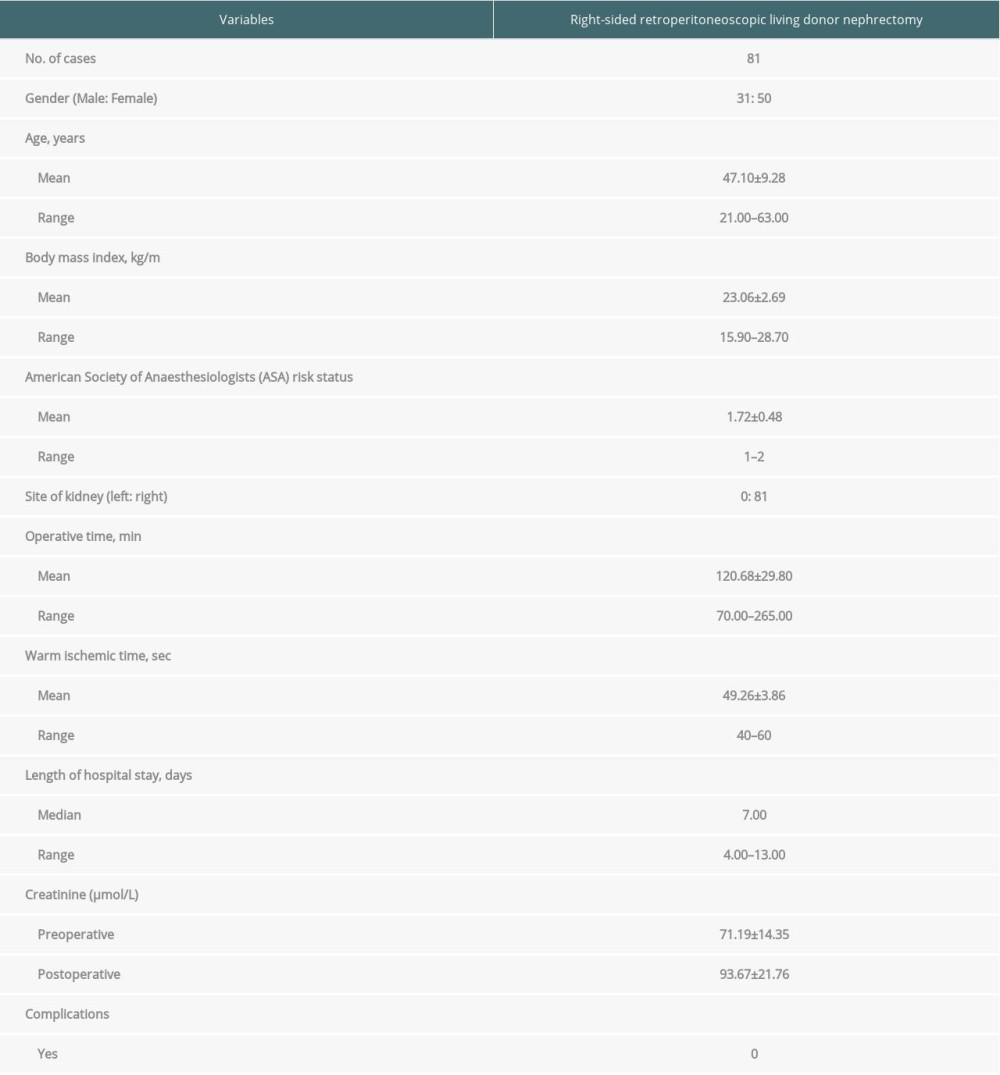
The present retrospective analysis included 81 donors who underwent right-sided retroperitoneoscopic LDN. They included 31 males and 50 females, with a mean age of 47.1 years (range 21–63 years). There were 61 parent-to-child donors, 5 spouse donors, and 15 sibling donors. Twenty donors and recipients had HLA mismatch in 1 locus, 22 donors and recipients had HLA mismatch in 2 loci, and 4 donors and recipients had HLA mismatch in 3 loci. The donors and recipients were fully matched in ABO blood type. Lymphocyte crossmatch between donors and recipients was negative (<10%), and the recipient population reactive antibody (PRA) was negative (<10%). The demographic and baseline characteristics of the donors are shown in Table 1.
OPERATIVE CHARACTERISTICS:
The mean operative time was 120.68±29.8 min. The mean warm ischemic time was 107.2±24.8 s. The estimate blood loss was 54.32 mL (range 50–400 mL). No transfusions were performed. Time to drainage tube removal was 3.31±0.82 days (range 2–6 days). The median length of hospital stay was 7 days (range 4–13 days). The preoperative creatinine was 71.19±14.35 mmol/L at baseline and 93.67±21.76 mmol/L postoperatively. There was no intraoperative conversion to open donor nephrectomy.
COMPLICATIONS:
There was neither intraoperative complication such as hemorrhage or lymph fistula nor kidney graft injury. There was no return to the operating theater and no readmissions within 30 days after discharge.
RECIPIENT OUTCOMES:
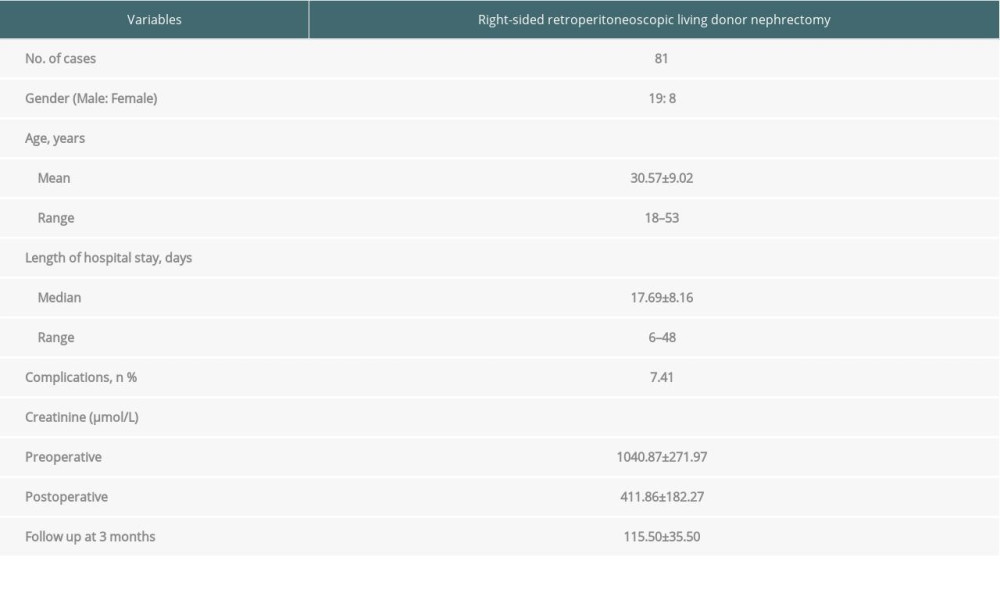
The demographic and baseline characteristics of the recipients are shown in Table 2. Right-sided retroperitoneoscopic living donor nephrectomy was uneventful in all recipients. Urine flow started 1–3 min following restoration of renal blood flow. Delayed graft function was reported in 2 cases. There was no graft renal vein thrombosis, ureteral stricture, or other complications. The recipients were followed up for duration of 3 months to 10 years. No graft rejection occurred.
Discussion
This retrospective single-center study demonstrated that right laparoscopic LDN is a safe and effective surgical procedure for liver donor kidney transplant. There were no major intraoperative or postoperative complications in donors or recipients. Kidney graft function rapidly recovered, with no graft loss reported.
Traditional live donor nephrectomy is open surgery, which has a long incision, is invasive, and has a slow pace of recovery, which, to a great extent, hampered the development of living-related renal transplant surgery. Clayman et al. [10] reported the first case of laparoscopic nephrectomy in 1991, ushering in the era of laparoscopic surgery. Four years later, Ratner et al. [11] completed the first transperitoneal laparoscopic live donor nephrectomy, which has since become popular at transplant centers because of its noninvasiveness, shortened length of hospital stay, faster recovery, and less steep learning curve
Renal transplant surgery is now widely performed in China, but the scarcity of donor kidneys has severely hampered the development of renal transplant surgery. As a result, living-related donor renal transplant has received increasing attention. Laparoscope LDN can be roughly categorized into laparoscopic peritoneal and laparoscopic retroperitoneal LDN. Most surgeons in China consider that laparoscopic retroperitoneal LDN causes less trauma to peritoneal organs and has a lower risk of postoperative gastrointestinal complications and peritoneal infections, while retroperitoneal LDN is only performed at a few transplant centers outside of China, as surgeons prefer the ample space and clear anatomic landmarks in transperitoneal laparoscopic surgery [15]. Currently, there is no evidence that transperitoneal LDN is superior to retroperitoneal LDN, and surgeons choose transperitoneal or retroperitoneal LDN mainly based on their technical dexterity. Our transplant center has performed laparoscopic living-related LDN since 2009 in 264 cases and has achieved satisfactory outcomes. No donor bleeding or deaths have occurred. Right retroperitoneal laparoscopic live donor nephrectomy is minimally invasive and is readily acceptable to donors as they suffer less pain and have a rapid recovery. Between April 2009 and February 2013, we performed right retroperitoneal laparoscopic live donor nephrectomy in all cases with excellent outcomes. The right kidney is lower than the left kidney, and subcostal transverse incision in right retroperitoneal laparoscopic live donor nephrectomy allows direct access to the surgical area. In addition, the excellent field of view permits relatively easy handling of the renal pedicle. Although the right renal vein is shorter, there is no confluence of the adrenal vein and gonadal vein, which facilitates handling of the right renal vein. Bachir et al. [16] found no significant difference in bleeding incidence and frequency of postoperative thrombosis in left
Conclusions
Right retroperitoneal laparoscopic live donor nephrectomy is safe and effective for renal transplant in living-related renal transplant by laparoscopic excision and extraction of the right kidney with vena cava flap.
Figures
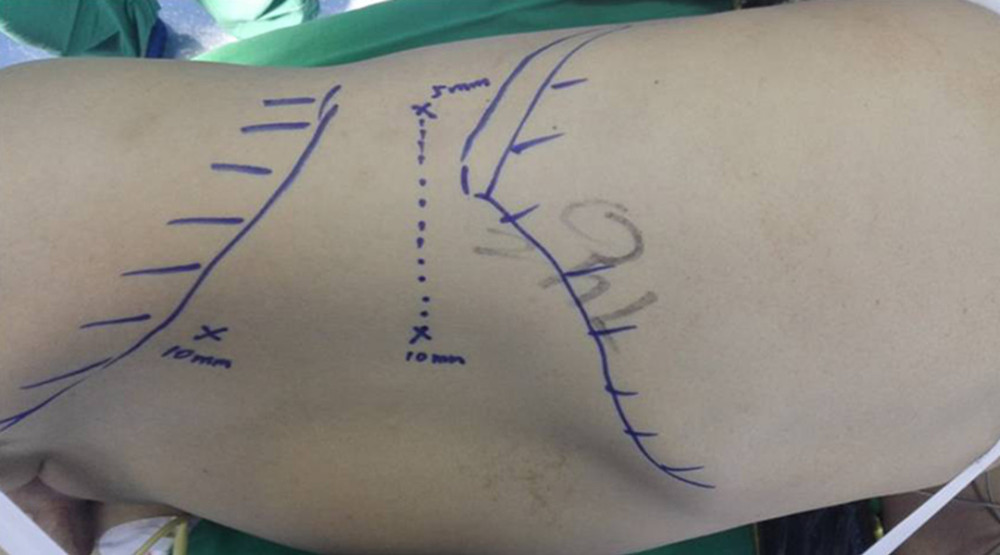 Figure 1. A 2-cm longitudinal incision is made 2 cm medial and superior to the right Anterior superior iliac spine. A 0.5-cm and 1.0-cm transverse incision are made in the posterior axillary line inferior to the 12th rib and in the anterior axillary line inferior to the 12th rib tip, respectively. Three ports are established. The distance is 6–8 cm between the 2 subcostal operating ports, facilitating the connection of the 2 ports during kidney extraction and controlling incision length.
Figure 1. A 2-cm longitudinal incision is made 2 cm medial and superior to the right Anterior superior iliac spine. A 0.5-cm and 1.0-cm transverse incision are made in the posterior axillary line inferior to the 12th rib and in the anterior axillary line inferior to the 12th rib tip, respectively. Three ports are established. The distance is 6–8 cm between the 2 subcostal operating ports, facilitating the connection of the 2 ports during kidney extraction and controlling incision length. 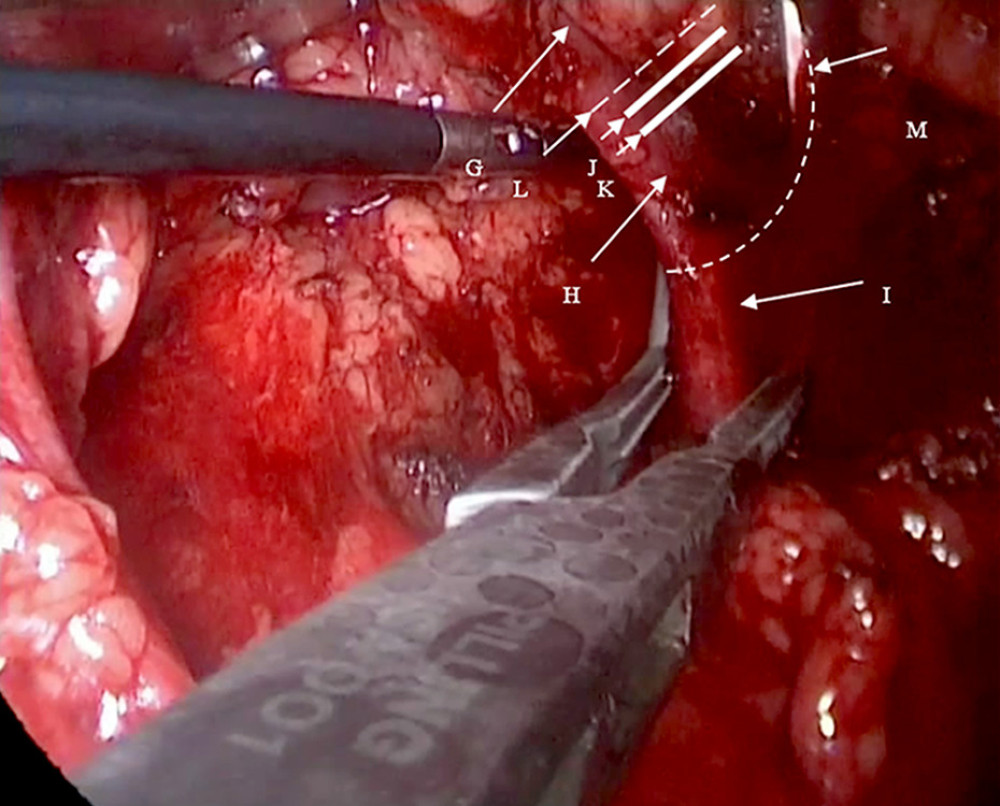 Figure 2. G: Right renal vein; H: the origin of the right renal vein; I: the inferior vena cava; J: the second Hem-o-lok; K: the first Hem-o-lok; L: the right renal vein is incised along the broken line for routine extraction of the right kidney. M: The renal vein is clamped at its origin in the direction of the inferior vena cava using vascular clamps or renal pedicle clamps and the renal vein is incised along the broken line. The origin of the right renal vein and partial inferior vena cava are preserved.
Figure 2. G: Right renal vein; H: the origin of the right renal vein; I: the inferior vena cava; J: the second Hem-o-lok; K: the first Hem-o-lok; L: the right renal vein is incised along the broken line for routine extraction of the right kidney. M: The renal vein is clamped at its origin in the direction of the inferior vena cava using vascular clamps or renal pedicle clamps and the renal vein is incised along the broken line. The origin of the right renal vein and partial inferior vena cava are preserved. 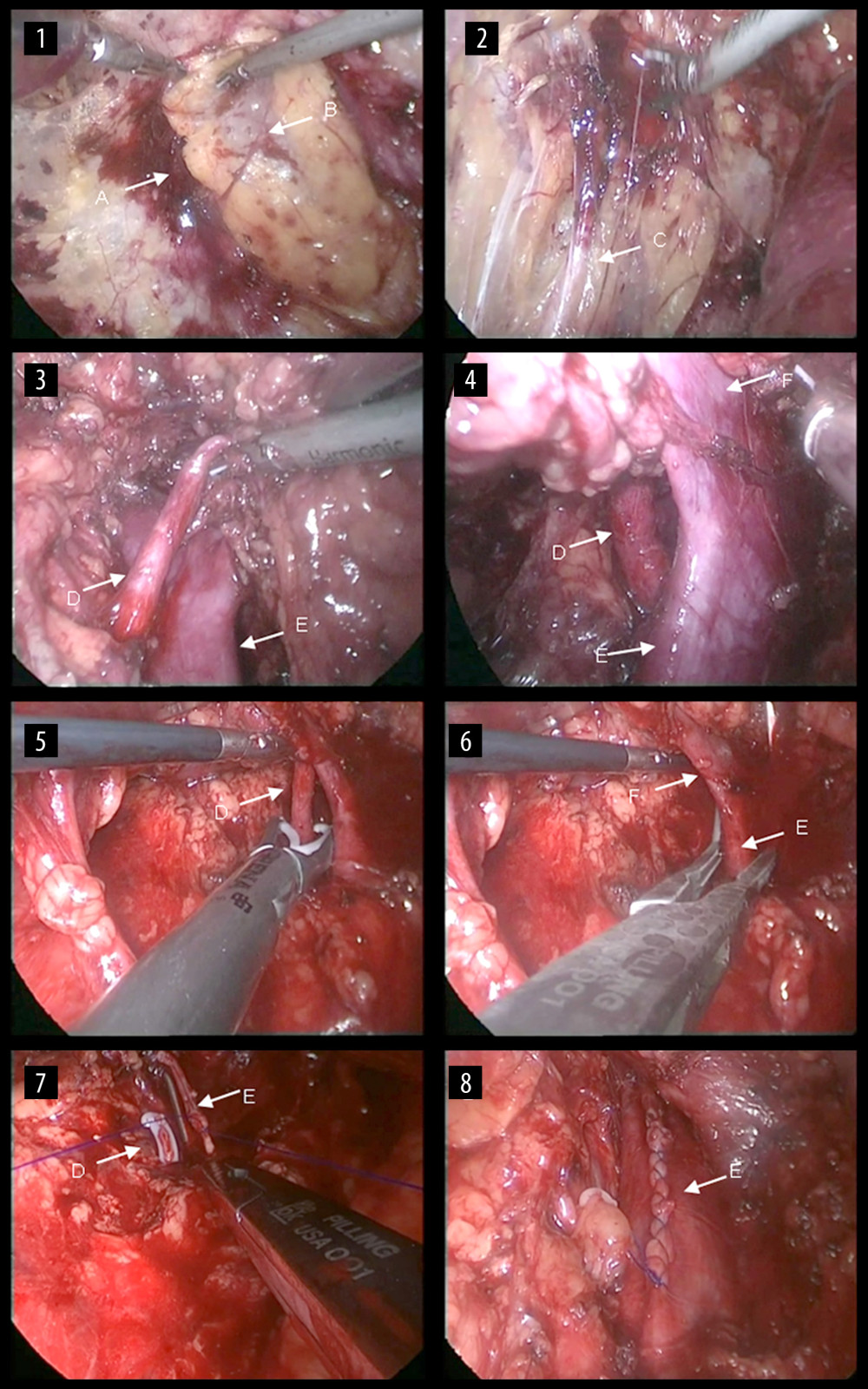 Figure 3. Surgical steps of right retroperitoneal laparoscopic live donor. nephrectomy. The renal fascia is opened (1), the ureter is fully dissociated (2), and then the right renal artery is dissected (3). The renal vein and the inferior vena cava are carefully dissected at their confluence (4). Then, the renal artery is clipped proximally (5), the renal vein is blocked at the confluence of the renal vein at the inferior vena cava and the renal vein is cut along with partial vena cava wall (6). The incision on the lateral wall of the inferior vena cava is then continuously sutured (7) and the vena cava after suturing is shown (8). A: the renal fascia; B: the right kidney; C: the ureter; D: the right renal artery; E: the inferior vena cava; F: the right renal vein.
Figure 3. Surgical steps of right retroperitoneal laparoscopic live donor. nephrectomy. The renal fascia is opened (1), the ureter is fully dissociated (2), and then the right renal artery is dissected (3). The renal vein and the inferior vena cava are carefully dissected at their confluence (4). Then, the renal artery is clipped proximally (5), the renal vein is blocked at the confluence of the renal vein at the inferior vena cava and the renal vein is cut along with partial vena cava wall (6). The incision on the lateral wall of the inferior vena cava is then continuously sutured (7) and the vena cava after suturing is shown (8). A: the renal fascia; B: the right kidney; C: the ureter; D: the right renal artery; E: the inferior vena cava; F: the right renal vein. References
1. Janki S, Dor FJ, Ijzermans JN, Surgical aspects of live kidney donation: An updated review: Front Biosci (Elite Ed), 2015; 7; 346-65
2. Ratner LE, Kavoussi LR, Sroka M, Laparoscopic assisted live donor nephrectomy – a comparison with the open approach: Transplantation, 1997; 63(2); 229-33
3. Ratner LE, Kavoussi LR, Chavin KD, Montgomery R, Laparoscopic live donor nephrectomy: Technical considerations and allograft vascular length: Transplantation, 1998; 65(12); 1657-58
4. Song G, Jeong IG, Kim YH, Kidney laterality and the safety of hand-assisted live donor nephrectomy: Review of 1000 consecutive cases at a single center: Urology, 2015; 85(6); 1360-66
5. Raschid MH, Francesco G, Olaf R, Right-sided transperitoneal hand-assisted laparoscopic donor nephrectomy: Is there an issue with the renal vessels?: J Endourol, 2010; 24(12); 1947-52
6. Qiu Y, Wang X, Song T, Comparison of both sides for retroperitoneal laparoscopic donor nephrectomy: Experience from a single center in China: Transplant Proc, 2017; 49(6); 1244-48
7. Delmonico FL, Dew MA, Living donor kidney transplantation in a global environment: Kidney Int, 2007; 71(7); 608-14
8. Dols L, Kok N, Alwayn IP, Laparoscopic donor nephrectomy: A plea for the right-sided approach: Transplantation, 2009; 87(5); 745-50
9. Mallon DH, Summers DM, Andrew JB, Pettigrew GJ, Defining delayed graft function after renal transplantation: Simplest is best: Transplantation, 2013; 96(10); 885-89
10. Clayman RV, Kavoussi LR, Soper NJ, Laparoscopic nephrectomy: Initial case report: J Urol, 1991; 146(2); 278-82
11. Ratner LE, Ciseck LJ, Moore RG, Laparoscopic live donor nephrectomy: Transplantation, 1995; 60(9); 1047-49
12. Wolf JS, Moon TD, Nakada SY, Hand-assisted laparoscopic nephrectomy: Technical considerations: Tech Urol, 1997; 3(3); 123-28
13. Giessing M, Reuter S, Deger S, Laparoscopic versus open donor nephrectomy in Germany: Impact on donor health-related quality of life and willingness to donate: Transplant Proc, 2005; 37(5); 2011-15
14. Dols LF, Kok NF, Ijzermans JN, Live donor nephrectomy: A review of evidence for surgical techniques: Transpl Int, 2010; 23(2); 121-30
15. Bachmannad A, Ruszat R, Giannini O, Retroperitoneoscopic donor nephrectomy: A retrospective, non-randomized comparison of early complications, donor and recipient outcome with the standard open approach: Eur Urol, 2005; 48(1); 90-96
16. Bachir BG, Hussein M, Nasr R, Evaluation of right versus left laparoscopic donor nephrectomy: Exp Clin Transplant, 2011; 9(5); 310-14
Figures
 Figure 1. A 2-cm longitudinal incision is made 2 cm medial and superior to the right Anterior superior iliac spine. A 0.5-cm and 1.0-cm transverse incision are made in the posterior axillary line inferior to the 12th rib and in the anterior axillary line inferior to the 12th rib tip, respectively. Three ports are established. The distance is 6–8 cm between the 2 subcostal operating ports, facilitating the connection of the 2 ports during kidney extraction and controlling incision length.
Figure 1. A 2-cm longitudinal incision is made 2 cm medial and superior to the right Anterior superior iliac spine. A 0.5-cm and 1.0-cm transverse incision are made in the posterior axillary line inferior to the 12th rib and in the anterior axillary line inferior to the 12th rib tip, respectively. Three ports are established. The distance is 6–8 cm between the 2 subcostal operating ports, facilitating the connection of the 2 ports during kidney extraction and controlling incision length. Figure 2. G: Right renal vein; H: the origin of the right renal vein; I: the inferior vena cava; J: the second Hem-o-lok; K: the first Hem-o-lok; L: the right renal vein is incised along the broken line for routine extraction of the right kidney. M: The renal vein is clamped at its origin in the direction of the inferior vena cava using vascular clamps or renal pedicle clamps and the renal vein is incised along the broken line. The origin of the right renal vein and partial inferior vena cava are preserved.
Figure 2. G: Right renal vein; H: the origin of the right renal vein; I: the inferior vena cava; J: the second Hem-o-lok; K: the first Hem-o-lok; L: the right renal vein is incised along the broken line for routine extraction of the right kidney. M: The renal vein is clamped at its origin in the direction of the inferior vena cava using vascular clamps or renal pedicle clamps and the renal vein is incised along the broken line. The origin of the right renal vein and partial inferior vena cava are preserved. Figure 3. Surgical steps of right retroperitoneal laparoscopic live donor. nephrectomy. The renal fascia is opened (1), the ureter is fully dissociated (2), and then the right renal artery is dissected (3). The renal vein and the inferior vena cava are carefully dissected at their confluence (4). Then, the renal artery is clipped proximally (5), the renal vein is blocked at the confluence of the renal vein at the inferior vena cava and the renal vein is cut along with partial vena cava wall (6). The incision on the lateral wall of the inferior vena cava is then continuously sutured (7) and the vena cava after suturing is shown (8). A: the renal fascia; B: the right kidney; C: the ureter; D: the right renal artery; E: the inferior vena cava; F: the right renal vein.
Figure 3. Surgical steps of right retroperitoneal laparoscopic live donor. nephrectomy. The renal fascia is opened (1), the ureter is fully dissociated (2), and then the right renal artery is dissected (3). The renal vein and the inferior vena cava are carefully dissected at their confluence (4). Then, the renal artery is clipped proximally (5), the renal vein is blocked at the confluence of the renal vein at the inferior vena cava and the renal vein is cut along with partial vena cava wall (6). The incision on the lateral wall of the inferior vena cava is then continuously sutured (7) and the vena cava after suturing is shown (8). A: the renal fascia; B: the right kidney; C: the ureter; D: the right renal artery; E: the inferior vena cava; F: the right renal vein. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860