16 September 2022: Review Paper
A Review of Humoral and Cellular Immune Responses to SARS-CoV-2 Vaccination Following Solid Organ Transplantation
Ilona Cieślak1ABCDEF, Magdalena Kwapisz2AEF*, Olga Tronina3ABCDEF, Joanna Gotlib1ABCDEF, Wojciech Lisik2ABCDEF, Dariusz Wasiak4ABCDEF, Marta Hreńczuk4ABCDEF, Mansur Rahnama5BDEF, Piotr Małkowski4ABCDEFDOI: 10.12659/AOT.936949
Ann Transplant 2022; 27:e936949
Abstract
ABSTRACT: The introduction of vaccines preventing a severe course of COVID-19 disease is particularly important in immunocompromised patients, among whom organ recipients and patients awaiting transplantation constitute a large group. The article is a critical review of 68 recent publications on the impact of the SARS-CoV-2 pandemic on transplantology worldwide. The study discusses research results concerning various aspects of SARS-CoV-2 vaccination in transplant patients; it also lists important factors influencing vaccination effectiveness. A suboptimal immune response to 2 doses of vaccine in this group of patients is a major challenge prompting further research. Therefore, this review aims to provide an update on the humoral and cellular immune responses to SARS-CoV-2 vaccination following solid organ transplantation.
Keywords: COVID-19, COVID-19 vaccine, transplant recipients, Immunocompromised Host, mRNA-1273 vaccine, mRNA Vaccine, COVID-19 Vaccines, Humans, Immunity, Cellular, Organ Transplantation, SARS-CoV-2, Vaccination, Viral Vaccines
Background
The first year of the COVID-19 pandemic resulted in a sharp decrease in the number of transplantations performed, both from deceased and living consenting donors. When comparing the number of transplantations carried out in the world year-on-year, the year 2020 saw a 17.5% decrease in the number of transplantations. This mostly involved kidney transplants (KTx) (decreased by 20.9%), pancreas transplants (decreased by 16.2%), lung transplants (decreased by 12.7%), liver transplants (LTx) (decreased by 11.3%), and heart transplants (decreased by 8%) [1]. In the United States, this resulted in an increase in patient mortality of up to 170% in patients awaiting transplantation, especially of the kidneys and lungs [2,3]. In Spain, the world leader in organ transplantation, the daily number of organ donations in the pandemic decreased from 7.2 to 1.2, and the number of transplantations performed decreased from 16.1 to 2.1 [4]. In Poland, in the beginning of the pandemic, the number of potential organ donors decreased by 43%, which resulted in a 60% reduction in the number of kidney and liver transplants performed [5]. The substantial decline in the number of transplantations was due to several factors. Firstly, there was concern about the life and health of patients from transplant waiting lists, as well as organ and bone marrow recipients, when exposed to SARS-CoV-2 infection. Transplantation centers in many countries recorded high mortality rates (up to 30%) caused by COVID-19 in this group of patients [6–11]. Secondly, limited access to intensive care units, where transplant patients would stay postoperatively and organ donors would be hospitalized. Moreover, for logistical reasons, constant testing for COVID-19 of both recipients and donors to monitor their infectious status was much more difficult [4,12–14]. To counter these very unfavorable tendencies, international transplant societies and transplantation centers developed guidelines to enable the continuation of organ transplantation. These activities, along with vaccinations against COVID-19 that began at the end of 2020, and gradually improved the situation [13–21]. The currently approved SARS-CoV-2 vaccines are BNT162b2 (Pfizer BioNTech) and mRNA-1273 (Moderna) containing mRNA encoding the S-glycoprotein (spike) of the virus [22,23] and 2 vector vaccines (ChAdOx1 nCoV-19 vaccine (AZD122) AstraZeneca and Ad26.COV2.S Janssen) containing replication-defective adenovirus (vector) with an integrated fragment of SARS-CoV-2 genetic material encoding the S-glycoprotein [24,25]. Although transplant recipients can safely receive any type of inactivated anti-SARS-CoV-2 vaccine, mRNA preparations are preferred for vaccination against COVID-19 in people with severe or moderate immunodeficiency [26]. Introduced at in 2021, COVID-19 mRNA vaccines BNT162b2 (Pfizer BioNTech) and mRNA-1273 (Moderna) offer 90–100% humoral and cellular immunity, preventing the acute form of the disease [22,23]. However, this does not apply to immunocompromised patients, including transplant recipients on immunosuppression. Therefore, this review aims to provide an update on the humoral and cellular immune responses to SARS-CoV-2 vaccination following solid organ transplantation.
Vaccinations in Solid Organ Transplant Recipients
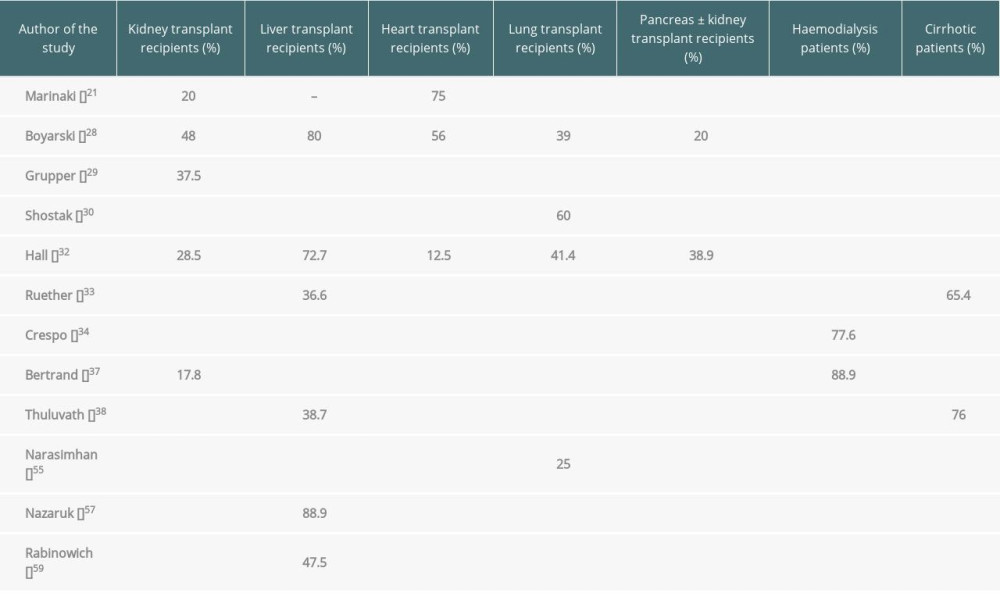
In 2 consecutive papers published in March and May 2021 in JAMA, Boyarsky demonstrated that on day 20 after the first dose of COVID-19 vaccination, antibodies were found in 17% of the examined transplant recipients, and on day 29 after the second dose of vaccination, antibodies were present in 54% of transplant recipients (48% of kidney transplants, 80% of liver transplants, 56% of heart transplants, 39% of lung transplants, and 20% of pancreas transplants). Thus, 46% of transplant recipients did not produce antibodies after 2 doses of mRNA vaccines [27,28]. Other researchers have also confirmed a worse response to vaccination in transplant patients, both in terms of humoral and cellular response. Immune response to 2 doses of vaccination was insufficient [21,29–32]; no immunity to infection was found in more than 40% of transplant recipients [21,29–32], especially in patients over 60 years of age, in whom the humoral response was only in 4.8% of the study participants [31]. In a study by Hall, involving 127 examined transplant patients, antibodies were found only in 5% of patients after the first dose and in 34.5% of patients after the second dose of mRNA-1273 (28.5% of kidney transplants, 72.7% of liver transplants, 12.5% of heart transplants, 41.4% of lung transplants, and 38.9% of pancreas with kidney transplants). Cellular response was found in 47.9% of patients [32]. In the author’s opinion, it may be the case that despite receiving 2 doses of vaccination, a significant proportion of transplant recipients will not produce a level of antibodies sufficient to protect themselves against infection, and in this group of patients an alternative treatment should be considered [32].
Vaccinations in Patients Awaiting Transplantation
A reduced response rate to vaccination was observed not only in organ recipients but also in patients awaiting transplantation: those receiving dialysis for renal failure and those with cirrhosis [33–36]. The dialysis patients and cirrhotic patients had a much weaker response to vaccination compared to the healthy general population, but better than those after KTx and LTx. As Crespo et al reported, positive response was obtained in 96.6% of healthy patients, 89.3% of patients on peritoneal dialysis, 77.6% of patients on haemodialysis, and 61.3% of KTx patients. The combined humoral and cellular immune response was 100% in healthy patients, 95.4% in dialysis patients, and 78.8% in kidney transplant patients [34]. As Bertrand noted, SARS-CoV-2 vaccination is more efficient in patients on dialysis therapy than KTx recipients, indicating that vaccination should be first recommended for those registered on a waiting list for kidney transplant [37]. In yet another study, a similarly positive response was found among patients awaiting liver transplant: 100% for healthy patients, 65.4% for cirrhotic patients, and 36.6% for LTx patients [33]. Thuluvath and colleagues found that 24% of those with chronic liver diseases had undetectable or suboptimal antibody responses, and 61.3% of liver transplant recipients had a poor response. Antibody levels were completely undetectable in 17.8% of liver transplant recipients, in 3.8% of those with cirrhosis, and in 4.3% of those with chronic liver diseases without cirrhosis [38] (Table 1).
The humoral immune response after 2 doses of the COVID-19 vaccine in 30–50% of KTx patients and 80–95% of dialysis patients was insufficient [28,33,39], and, according to French authors, it is an indication for the administration of a third dose of the vaccine [36]. De Belo presented the results of a study on 396 transplant patients in whom the administration of the third dose increased the humoral response rate from 46.3% to 67.9%. In addition, in more than 40% of patients in whom there was no seroconversion despite the second dose, the third dose resulted in a humoral response [40]. Numerous authors have reported an improvement in the humoral response in organ recipients after the third dose of the vaccine [41–45], but it was also reported to be ineffective by others [32,33,46]. In view of this, Hall’s proposal of alternative treatment in patients without established seroconversion, despite vaccination, may be legitimate [32]. Possibly, the introduction of the fourth and fifth doses of vaccination in these patients might also be a solution [47,48].
Factors Influencing Response to Vaccination
STATUS POST-COVID-19 INFECTION:
It has been observed that after the first dose of the vaccine, post-transplant patients who recovered from COVID-19 had antibody levels similar to the healthy, immunocompetent population [31,49–51]. This pattern was noted in both renal and hepatic transplant patients as well as in dialysis patients awaiting transplantation [31,49–52]. The humoral immune response was better in patients after a full-blown symptomatic course of infection and was found in 68.4% of patients, whereas seropositive results after a mild or asymptomatic course of infection were found in 9.4% and 4.6% of patients, respectively [31]. Despite the increase in immunity among organ recipients who recovered from COVID-19, most researchers agree that for the level of humoral response to be sufficient, 2 doses of the SARS-CoV-2 vaccine are required [49–51].
TYPE OF VACCINE USED AND PATIENT AGE:
The available recommended COVID-19 vaccines – BNT162b2 (Pfizer BioNTech) and mRNA-1273 (Moderna) – are highly effective in preventing acute course of the disease [22,23]. Nevertheless, more recent studies of a healthy population have revealed a slightly higher effectiveness of the Moderna vaccine [53,54]. Similar results have been obtained in a study of lung transplant patients, in whom the humoral response after 2 doses of vaccination was slightly higher after the use of mRNA-1273 compared to the BNT162b2 vaccine [55]. The serological response to vaccination is also related to patient age. When examining a group of healthcare workers vaccinated with 2 doses, Richards found significantly lower levels of antibodies in people over 50 years of age than in the younger group after use of the mRNA-1273 and BNT162b2 vaccines [56]. A similar pattern was observed after vaccination of kidney, liver, and other organ recipients [31,32,46,57].
TYPE OF ORGAN TRANSPLANT AND IMMUNOSUPPRESSION USED:
It is believed that the liver is more immunologically privileged than other organs, thus it requires slightly less immunosuppression and develops a better response to vaccination. Herrera reported cellular and humoral responses in 90% of 56 liver transplant recipients who received 2 doses of the Moderna vaccine [58]. In a study by Nazaruk, humoral response was found in 88.9% of 61 LTx patients vaccinated with 2 doses of the Pfizer vaccine [57]. However, in a study by Ruether and Rabbinowich on the effect of vaccination in LTx patients, a humoral response was found only in 36.6% and 47.5% of cases, respectively [33,59]. According to most researchers, recipients of other organs, especially the kidneys and lungs, develop a weaker response to vaccination. Humoral response does not usually exceed 30–60% in KTx patients [29,34,36,57] and 25% in lung transplant patients [55]. Although research points to a lower effectiveness of vaccination in kidney and lung recipients [29,31,34,36,55], it is known that vaccination effectiveness depends on a variety of factors. In addition to older patient age [31–33,46,57], research shows the importance of other pathologies reducing vaccine effectiveness of vaccines in patients with hypogammaglobulinemia [58], abnormal function of the transplanted organ [45], a white blood cell count lower than 1500 cells [46], diabetes, antithymocytic globulin treatment in the year preceding vaccination [60], and hypertension [33]. The vast majority of researchers claim that the use of immunosuppressants has had the greatest impact on the recorded vaccination failure. Mycophenolate mofetil [45,46,61] and belatacept [46,61] significantly reduce the serological response to vaccination. The latter, when used after kidney transplantation, reduces response to vaccination to 5%, while in kidney recipients who were not administered this medication, the response was as high as 50% [36,46,62]. In addition, vaccination in the first year after transplantation [58], administration of high doses in triple immunosuppressive regimens [46,63], or administration of steroids [61] significantly reduce the serological response to vaccination. There have been attempts to modify immunosuppression, mainly through discontinuation of mycophenolate mofetil, in those organ recipients in whom due to the lack of response to the third dose of the vaccine, the administration of a fourth or even fifth dose is considered [47,48]. Netti reported a positive effect, improving the humoral and cellular response, of m-Tor inhibitors used in immunosuppression in kidney recipients [64].
POST-VACCINATION ADVERSE EVENTS:
There is no information available on severe post-COVID-19 vaccination-associated complications in transplant patients [40,44,46,47,58,60,61,65,66]. There have been no cases of the presence of donor-specific antibodies (DSA) that could damage the transplanted organ [58,60,67,68]. In a literature review on the efficacy and safety of the administration of the third dose of the vaccine based on data from 835 organ recipients, Efros did not report any cases of anaphylactic shock or other life-threatening complications. Typical adverse effects were mild or moderate pain at the injection site, headache, and short-term general weakness. In 1 case, mild rejection symptoms, not requiring intensification of immunosuppression, were observed on day 7 after the third dose of vaccine. According to the researchers, the relationship between this incident and vaccination is only hypothetical [44,46].
It is widely known that patient immunity after the second dose of vaccine is insufficient [28,33,39]; hence, the introduction of the third dose, also not always effective [32,33,46]. In view of this, Hall’s proposal of alternative treatment in patients without established seroconversion, despite vaccination, may be legitimate [32]. Possibly, the introduction of the fourth and fifth doses of vaccine in these patients might also be a solution [47,48].
Conclusions
The aim of the above is to review the available literature on the impact of the SARS-CoV-2 pandemic on organ transplantation worldwide. The introduction of vaccines preventing a severe course of COVID-19 is particularly important in immunocompromised patients, among whom organ recipients and patients awaiting transplantation constitute a large group. The fact that there appeared so many scientific papers based on post-transplant research in such a short period of time contributed to transplantation safety. However, the suboptimal response to vaccinations in this group of patients is a major challenge for both doctors and the patients themselves. Further prospective studies assessing the response to vaccination, antibodies levels, or COVID-19 incidence despite vaccination will certainly bring new data and provide answers to many questions.
References
1. Kute VB, Tullius SG, Rane H, Global impact of the COVID-19 pandemic on Solid organ transplantation: Transplant Proc, 2022 [Online ahead of print]
2. Cholankeril G, Podboy A, Alshuwaykh O, Early impact of COVID-19 on solid organ transplantation in the United States: Transplantation, 2020; 104(11); 2221-24
3. DeFilippis E, Farr MA, Givertz MM, Challenges in heart transplantation in the era of COVID-19: Circulation, 2020; 141(25); 2048-51
4. Domínguez-Gil B, Coll E, Fernández-Ruiz M, COVID-19 in Spain:Transplantation in the midst of the pandemic: Am J Transplant, 2020; 20(9); 2593-98
5. Kwapisz M, Małkowski P, Tronina O, Effects of the COVID-19 pandemic on solid organ transplantation during 2020 in Poland compared with countries in Western Europe, Asia, and North America:A review: Med Sci Monit, 2021; 27; e932025
6. Watanabe M, Yakushijin K, Funakoshi Y, The safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in Japanese patients after allogeneic stem cell transplantation: Vaccines (Basel), 2022; 10(2); 158
7. Clarke C, Lucisano C, Prendecki M, Informing the risk of kidney transplantation versus remaining on the waitlist in the COVID-19 era: Kidney Int Rep, 2021; 6(1); 46-55
8. Caillard S, Anglicheau D, Matignon M, An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants: Kidney Int Dec, 2020; 98(6); 1549-58
9. Mamode N, Ahmed Z, Jones G, Mortality rates in transplant recipients and transplantation candidates in a high prevalence COVID-19 environment: Transplantation, 2021; 105(1); 212-15
10. Alberici F, Delbarba E, Manenti Ch, Management of patients on dialysis and with kidney transplantation during the SARS-CoV-2 (COVID-19) pandemic in Brescia, Italy: Kidney Int Rep, 2020; 5(5); 580-85
11. Lentine KL, Vest LS, Schnitzler MA, Survey of US living kidney donation and transplantation practices in the COVID-19 era: Kidney Int Rep, 2020; 5(11); 1894-905
12. Díaz-Reixa J, Domínguez-Gil B, Coll E, Renal transplantation during COVID-19 period in Spain: Arch Esp Urol, 2020; 73(5); 447-54
13. American Society of Transplantation: SARS-CoV-2 (Coronavirus, 2019-nCoV): Recommendations and guidance for organ donor testing updated on October 5 2020 https://www.myast.org/recommendations-and-guidance-organ-donor-testing
14. Trubin PA, Azar MM, Malinis M, Diagnostic testing of COVID-19 in solid organ transplantation:Current clinical application and future strategies: Curr Transpl Rep, 2020; 7; 390-98
15. Kumar D, Manuel O, Natori Y, COVID-19:A global transplant perspective on successfully navigating a pandemic: Am J Transplant, 2020; 20(7); 1773-79
16. Dahlqvist G, Ciccarelli O, Van Vlierberghe H, Liver transplantation during the COVID-19 epidemic:Recommendations from the Belgian Liver Intestine Transplant Committee (BeLIAC): Acta Gastro-Enterologica Belgica, 2020; LXXXIII; 340-43
17. Weiss M, Lalani J, Patriquin-Stoner Ch, Summary of international recommendations for donation and transplantation programs during the coronavirus disease pandemic: Transplantation, 2021; 105(1); 14-17
18. Organización Nacional de Trasplantes: Spanish recommendations to manage organ donation and transplantation regarding the infection associated with the new corona-virus (Sars-Cov-2) producer of Covid-19 Available from: []https://content.tts.org/content/covid19/COVID-19-Summary-of-Spanish-recommendations-on-organ-donation-and-transplantation20200413.pdf
19. Galvan NTN, Moreno NF, Garza JE, Donor and transplant candidate selection for solid organ transplantation during the COVID-19 pandemic: Am J Transplant, 2020; 20; 3113-22
20. The Transplantation Society Transplant Infectious Diseases Section: Guidance on coronavirus disease 2019 (COVID-19) for transplant clinicians Available from:https://tts.org/index.php?option=com_content&view=article&id=749&Itemid=140
21. Marinaki S, Adamopoulos S, Degiannis D, Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients: Am J Transplant, 2021; 21(8); 2913-15
22. Polack FP, Thomas SJ, Kitchin N, Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine: N Engl J Med, 2020; 383; 2603-15
23. Baden LR, El Sahly HM, Essink B, Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine: N Engl J Med, 2021; 384; 403-16
24. Voysey M, Clemens SAC, Madhi SAOxford COVID Vaccine Trial Group, Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2:An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK: Lancet, 2021; 397(10269); 99-111 Erratum in:Lancet. 2021;397(10269):98
25. Sadoff J, Gray G, Vandebosch A, Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19: N Engl J Med, 2021; 384(23); 2187-201
26. https//www.mp.pl/szczepienia/artykuly/wytyczne/266040,wytyczne-szczepienia-przeciwko-covid-19-tymczasowe-zalecenia-centers-for-disease-control-and-prevention-stan-na-31-marca-2022-r#szczepienie-COVID-19-osob-z-ciezkim-umiarkowanym-niedoborem-odpornosci
27. Boyarsky BJ, Werbel WA, Avery RK, Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients: JAMA, 2021; 325; 1784-86
28. Boyarsky BJ, Werbel WA, Avery RK, Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients: JAMA, 2021; 325; 2204-6
29. Grupper A, Rabinowich L, Schwartz D, Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus: Arab Archaeol Epigr, 2021; 21; 2719-26
30. Shostak Y, Shafran N, Heching M, Early humoral response among lung transplant recipients vaccinated with BNT162b2 vaccine: Lancet Respir Med, 2021; 9; e52-e53
31. Cristelli MP, Viana LA, Fortaleza CM, Lower seroprevalence for SARS-CoV-2-specific antibodies among kidney transplant recipients compared to the general population in the city of Sao Paulo, Brazil: Transpl Infect Dis, 2021; 23(5); e13706
32. Hall VG, Ferreira VH, Ierullo M, Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients: Am J Transplant, 2021; 21; 3980-89
33. Ruether DF, Schaub GM, Duengelhoef PM, SARS-CoV2-specific humoral and T-cell immune response after second vaccination in liver cirrhosis and transplant patients: Clin Gastroenterol Hepatol, 2022; 20(1); 162-72
34. Crespo M, Barrilado-Jackson A, Padilla E, Negative immune responses to two-dose mRNA COVID-19 vaccines in renal allograft recipients assessed with simple antibody and interferon gamma release assay cellular monitoring: Am J Transplant, 2022; 22(3); 786-800
35. Simon B, Rubey H, Treipl A, Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared with healthy controls: Nephrol Dial Transplant, 2021; 36(9); 1709-16
36. Sakhi H, Chavarot N, Attias P, COVID-19 vaccination in dialysis and kidney transplant patients, 2021; 17(4); 208-13
37. Bertrand D, Hamzaoui M, Lemée V, Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients: J Am Soc Nephrol, 2021; 32(9); 2147-52
38. Thuluvath PJ, Robarts P, Chauhan M, Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases: J Hepatol Dec, 2021; 75(6); 1434-39
39. Grupper A, Katchman H, SARS-CoV-2 vaccines:Safety and immunogenicity in solid organ transplant recipients and strategies for improving vaccine responses: Curr Transplant Rep, 2022; 9(1); 35-47
40. Del Bello A, Abravanel F, Marion O, Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients, 2022; 22(1); 322-23
41. Kamar N, Abravanel F, Marion O, Three doses of an mRNA Covid 19 vaccine in solid organ transplant recipients: N Engl J Med, 2021; 385; 661-62
42. Masset C, Kerleau C, Garandeau C, A third injection of the BNT162b2 mRNA COVID-19 vaccine in kidney transplant recipients improves the humoral immune response: Kidney Int, 2021; 100; 1132-35
43. Bar-On YM, Goldberg Y, Mandel M, Protection of BNT162b2 vaccine booster against COVID-19 in Israel: N Engl J Med, 2021; 385; 1393-400
44. Werbel WA, Boyarsky BJ, Ou MT, Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients:A case series: Ann Intern Med, 2021; 174; 1330-32
45. Peled Y, Ram E, Lavee J, Third dose of the BNT162b2 vaccine in heart transplant recipients:Immunogenicity and clinical experience: J Heart Lung Transplant, 2022; 41(2); 148-57
46. Efros O, Anteby R, Halfon M, Efficacy and safety of third dose of the COVID-19 vaccine among solid organ transplant recipients:A systemic review and meta-analysis: Vaccines (Basel), 2022; 10(1); 95
47. Tang K, Wu X, Luo Y, Meta-analysis of immunologic response after COVID-19 mRNA vaccination in solid organ transplant recipients: J Infect, 2022; 84(5); e73-e75
48. Abedon AT, Teles MS, Alejo JL, Improved antibody response after a fifth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients:A case series: Transplantation, 2022; 106(5); e262-e63
49. Lemieux JE, Li A, Gentili M, Perugino CA, Vaccine serologic responses among transplant patients associate with COVID-19 infection and T peripheral helper cells: MedRxiv, 2021; 2021; 21260338
50. Demonbreun AR, Sancilio A, Velez MP, Comparison of IgG and neutralizing antibody responses after one or two doses of COVID-19 mRNA vaccine in previously infected and uninfected individuals: E Clinical Medicine, 2021; 38; 101018
51. Caballero-Marcos A, Citores MJ, Alonso-Fernández R, Decreased long-term SARS-CoV-2-specific humoral immunity in liver transplant recipients 12-months after COVID-19: Liver Transpl, 2021; 28(6); 1039-50
52. Speer C, Morath Ch, Töllner M, Humoral responses to single-dose BNT162b2 mRNA vaccination in dialysis patients previously infected with SARS-CoV-2: Front Med (Lausanne), 2021; 8; 721286
53. Self WH, Tenforde MW, Rhoads JP, Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions – United States, March–August 2021: MMWR Morb Mortal Wkly Rep, 2021; 70(38); 1337-43
54. Steensels D, Pierlet N, Panders J, Comparison of SARS-Cov2 antibody response following vaccination with BNT162b2 and mRNA-1273: JAMA, 2021; 326(15); 1533-35
55. Narasimhan M, Mahimainathan L, Clark AE, Serological response in lung transplant recipients after two doses of SARS-CoV-2 mRNA vaccines: Vaccines (Basel), 2021; 9(7); 708
56. Richards NE, Keshavarz B, Workman LJ, Comparison of SASRS-CoV-2 antibody response by age among recipients of the BNT162b2 vs the mRNA-1273 vaccine: JAMA Network Open, 2021; 4(9); e2124331
57. Nazaruk P, Monticolo M, Jędrzejczak AM, Unexpectedly high efficacy of SARS-CoV-2 BNT162b2 vaccine in liver versus kidney transplant recipients – is it related to immunosuppression only?: Vaccines (Basel), 2021; 9(12); 1454
58. Herrera S, Colmenero J, Pascal M, Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients: Am J Transplant, 2021; 21(12); 3971-79
59. Rabinowich L, Grupper A, Baruch R, Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients: J Hepatol, 2021; 75; 435-38
60. Cucchiari D, Egri N, Bodro M, Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients: Am J Transplant, 2021; 21(8); 2727-39
61. Chavarot N, Morel A, Leruez-Ville M, Weak antibody response to three doses of mRNA vaccine in kidney transplant recipients treated with belatacept: Am J Transplant, 2021; 21(12); 4043-51
62. Ou MT, Boyarsky BT, Chiang TPY, Immunogenicity and reactogenicity after SARS-CoV-2 mRNA vaccination in kidney transplant recipients taking belatacept: Transplantation, 2021; 105(9); 2119-23
63. Benotmane I, Gautier G, Perrin P, Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses: JAMA, 2021; 326; 1063
64. Netti GS, Infante B, Troise D, mTOR inhibitors improve both humoral and cellular response to SARS-CoV-2 messenger RNA BNT16b2 vaccine in kidney transplant recipients: Am J Transplant, 2022; 22(5); 1475-82
65. Westhoff TH, Seibert FS, Anft M, Third vaccine dose substantially improves humoral and cellular SARS-CoV-2 immunity in renal transplant recipients with primary humoral nonresponse: Kidney Int, 2021; 100; 1135-36
66. Hall VG, Ferreira VH, Ku T, Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients: N Engl J Med, 2021; 385; 1244-46
67. Boyarsky BJ, Ou MT, Greenberg RS, Safety of the first dose of SARS-CoV-2 vaccination in solid organ transplant recipients: Transplantation, 2021; 105; e56-57
68. Phadke VK, Scanlon N, Jordan SC, Immune responses to SARS-CoV-2 in solid organ transplant recipients: Curr Transplant Rep, 2021; 8; 127-39
In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860