01 February 2022: Original Paper
Effects of Substance P for Liver Regeneration in Rat Hepatectomy Models: A Preliminary Experimental Animal Study
Hyunmin KoDOI: 10.12659/AOT.934801
Ann Transplant 2022; 27:e934801
Abstract
BACKGROUND: This experiment was designed to investigate the effect of substance P (SP) on liver regeneration after partial hepatectomy in a rat model.
MATERIAL AND METHODS: Male Sprague-Dawley rats were used in this experiment and were divided into an untreated sham group (n=3); a saline group (control group) given a saline injection after hepatectomy (n=4); and an SP group (experimental group) given SP injection after hepatectomy (n=4). The experiments were repeated 3 times in the sham, saline, and SP groups (days 1, 2, and 3). Liver function tests were performed on the serum. Immunohistochemistry was performed for proliferating cell nuclear antigen (PCNA), Ki-67, and CD133, and western blotting was performed for PI3KC, Akt, p38, ERK, and mTOR.
RESULTS: Liver function test results indicated higher levels in the saline group than in the SP group at day 1. However, the difference gradually disappeared at days 2 and 3. PCNA and Ki-67 were more highly expressed in the SP group than in the saline and sham groups at day 1. CD133 was strongly expressed in the SP group at day 2 compared to that in the sham and saline groups. The changes in mTOR/Akt/PI3KC pathway protein and ERK/p38 signaling protein expression levels were not significantly different among groups.
CONCLUSIONS: SP accelerated the regeneration stage after hepatectomy in a rat model.
Keywords: Liver Regeneration, Liver Transplantation, substance P, Animals, Hepatectomy, Liver Function Tests, Male, Rats, Rats, Sprague-Dawley
Background
Although hepatectomy outcomes have markedly improved in recent decades, hepatic damage is inevitable during liver resection and transplantation. Hepatic failure after liver resection is a life-threatening complication [1]. In living-donor liver transplantation, it is necessary to minimize the size of the graft for donor safety. However, the graft size is related to the recipient’s prognosis. The post-hepatectomy liver failure can be prevented by promoting the liver regeneration process [2]. Therefore, liver regeneration is a very important prognostic factor in clinical settings, including liver resection and liver transplantation.
Substance P (SP) acts as a sensory neurotransmitter and neuromodulator of pain information in the central nervous system [3]. SP mobilizes bone marrow mesenchymal stem cells into peripheral blood, and promotes their migration to, and thereby the regeneration of, damaged tissues; it has been reported that self-healing mechanisms recruit bone marrow stem cells. SP is synthesized and secreted by peripheral organs such as the liver and lungs. SP controls a variety of physiological and pathophysiological functions, including inflammation, proliferation, cell excitability, and anti-apoptosis, by binding to the high-affinity neurokinin-1 receptor [4–6]. The functions of SP include vasodilation, activation of immune cells, regulation of pain transmission, and stimulation of cell growth in normal and cancer cells. SP is also produced by immune cells and acts in either an autocrine or paracrine manner to regulate their function, and has been reported to promote ocular inflammation, wound healing, and tissue homeostasis [7]. SP-induced cytokines promote the multiplication of cells needed for the repair, replacement, and growth of new blood vessels [8].
This study was designed to demonstrate the effect of SP on regeneration after partial hepatectomy in rats. In addition, the PI3K/Akt/mTOR pathway, which plays an important role in early regeneration after liver resection, was evaluated to analyze the mechanism of SP [9]. Molecules involved in the actions of cell proliferation were evaluated [10]. CD133 was analyzed to evaluate the ability of SP to mobilize endothelial progenitor cells (EPC) [11].
Material and Methods
ANIMAL:
Eight-week-old Sprague-Dawley rats were used (Young Bio Co., Seoul, Korea). The animals were maintained on a 12-h light/dark cycle and fed standard chow. All experiments were approved by the Kyung Hee Medical Animal Care Committee and were conducted according to the principles and procedures outlined in the NIH Guide for the Care and Use of Laboratory Animals.
EXPERIMENTAL PROTOCOLS:
The animals were randomly divided into 3 groups: an untreated sham group (n=3), a saline group (control group) given saline injection after hepatectomy (n=4), and an SP group (experimental group) given SP injection after hepatectomy (n=4). The experiments were repeated 3 times in the sham, saline, and SP groups (days 1, 2, and 3). In the SP group, 5 nmol/kg of SP was injected through the tail vein.
Anesthesia was induced with a mixture of oxygen (2 L/min) and N2O (0.5 L/min) containing 2% isoflurane. Light pressing of both ribs at the back exposed 70% of the lobes. Then, the surrounding ligaments were peeled off so that only the lobes could move. Next, 3-0 silk was used to bind the blood vessels and parenchyma of the lobes. The lobes were then cut with scissors and closed (Figure 1).
LIVER FUNCTION TESTS IN THE SERUM:
Serum was isolated from the whole blood by centrifugation (2000 rpm, 10 min) after coagulation. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin levels were determined using a biochemical analyzer at the Korean Animal Medical Science Institute.
TISSUE PREPARATION:
Livers were enucleated under anesthesia with a mixture of oxygen (2 L/min) and N2O (0.5 L/min) containing 4% isoflurane and immersed in 4% formaldehyde in 0.1 M phosphate-buffered saline (PBS) at pH 7.4. Whole livers were dissected and immersed in the same fixative for 8 h. After fixation, a paraffin block was prepared after dehydration processing for immunohistochemistry; it was flash-frozen in liquid nitrogen and preserved at -80°C for western blotting.
IMMUNOHISTOCHEMISTRY:
Liver samples were trimmed out from the central portion and rinsed with 0.01 M PBS at pH 7.4. After thorough rinsing, the liver pieces were embedded in paraffin and cut into 5-μm-thick sections. To block nonspecific binding sites, the sections were treated with the VECTASTAIN ABC Kit (Vector Laboratories, Burlingame, CA, USA). The sections were incubated at 4°C with antibodies against proliferating cell nuclear antigen (PCNA; Abcam, Cambridge, MA, USA; dilution 1: 1000), Ki-67 (Abcam; dilution 1: 1000), and CD133 (Abcam; dilution 1: 500). Thereafter, the sections were incubated with the appropriate biotinylated secondary antibody (dilution 1: 200) and then visualized using the ImmPACT NovaRED peroxidase substrate. After rinsing in PBS, stained hematoxylin sections were mounted on glass slides with a mounting medium.
WESTERN BLOT ANALYSIS:
Tissues were homogenized and lysed using a lysis buffer containing 1 mM PMSF (Cell Signaling Technology, Boston, MA, USA). Protein concentration was determined using a BCA protein assay (Thermo Fisher Scientific, Rockford, IL, USA) according to the manufacturer’s protocol. Thirty micrograms of protein were fractionated using ~6–12% SDS-PAGE and transferred via electrophoresis to nitrocellulose blotting membranes. The membranes were blocked with 1% bovine serum albumin for 1 h at room temperature and then incubated overnight at 4°C with antibodies against PI3KC (Santa Cruz Biotechnology, CA, USA), p-PI3KC (Abcam), mTOR, p-mTOR, Akt, p-Akt, ERK, p-ERK, p38, p-p38 (Cell Signaling Technology), and β-actin (Sigma-Aldrich, St. Louis, MO, USA), which were diluted at 1: 1000 (1: 5000 for β-actin) in Tris-buffered saline containing 0.05% Tween-20 (TBS-T). The signal intensity of each protein band was quantified using ImageJ software (NIH, USA).
STATISTICAL ANALYSIS:
All data are presented as mean±standard deviation (SD). Statistical analyses were carried out with one-way analysis of variance, and significant data were examined by Tukey’s post hoc test using GraphPad Prism 5 software (GraphPad Software, Inc., San Diego, CA, USA). Immunohistochemistry images were quantitatively calculated by western blot and cell counting. *
Results
LIVER FUNCTION TEST IN SERUM:
Results of the liver function tests in serum were analyzed for AST, ALT, and total bilirubin in each group on days 1, 2, and 3. AST, ALT, and total bilirubin levels were significantly higher in the saline group than in the SP group at day 1 after hepatectomy. However, the difference gradually disappeared at days 2 and 3 (Figure 2).
IMMUNOHISTOCHEMISTRY OF PCNA AND KI-67:
To analyze the differences in liver regeneration after liver resection between each group, immunohistochemistry of PCNA and Ki-67 was performed. PCNA was more highly expressed in the SP group than in the saline and sham groups at day 1. However, no differences were observed at days 2 and 3 (Figure 3A). Similarly, the expression of Ki-67 was higher in the SP group than in the saline and sham groups at day 1, but there was no difference at days 2 and 3 (Figure 3B).
IMMUNOHISTOCHEMISTRY OF CD133:
To analyze the differences in stem cell mobilization after liver resection between each group, immunohistochemistry of CD133 was performed. CD133 was not different between the groups at days 1 and 3, but was more strongly expressed in the SP group at day 2 compared to that in the sham and saline groups (Figure 3C).
EXPRESSION OF MTOR/AKT/PI3KC PATHWAY PROTEINS AND MAPK SIGNALING PROTEINS:
In this study, changes in the activation state of signaling proteins upon SP injection after hepatectomy were investigated. Immunoblot analysis was performed to evaluate the regulation of mTOR, Akt, PI3KC, and MAPK signaling proteins. A comparative analysis of protein expression in the saline and SP groups after liver resection showed that the phosphorylation levels of mTOR and Akt proteins were higher in the SP group than in the saline group. The phosphorylation level of PI3KC protein was lower at day 1, but it was slightly higher at days 2 and 3 in the SP group than in the saline group (Figure 4).
The phosphorylation level of ERK protein was higher in the SP group than in the saline group at days 1, 2, and 3. In contrast, the phosphorylation level of p38 protein increased at day 1, but decreased at day 2 in the SP group (Figure 5).
Discussion
Liver regeneration after partial hepatectomy is a complex and well-orchestrated phenomenon [12]. Interleukin-6, tumor necrosis factor-α, hepatocyte growth factor, epidermal growth factor, and thyroid hormones are known to regulate regeneration in the normal liver [13,14].
In our data, the lower levels of liver function test in the SP group than in the saline group at day 1 demonstrate that SP protects against deterioration of liver function. Serum liver function test results were also consistent with PCNA and Ki-67 expression levels. The immune reactivity of PCNA and Ki-67 is commonly used to assess proliferative activity in normal, regenerative, and neoplastic livers in rodents and humans [15,16]. Under normal liver regeneration conditions, PCNA protein levels during rat liver regeneration after 70% partial hepatectomy peaked at 36–48 h [17]. Seven to 10 days after hepatectomy, the liver regenerated to 93% of its normal size, and 20 days after hepatectomy, the liver completely recovered its initial volume [18]. In the SP group, PCNA and Ki-67 expression levels peaked at day 1 (24 h). This result suggests that when SP is injected, the process of regeneration proceeds faster than in the normal liver regeneration process.
Some studies have reported that SP stimulates stem cells. An “endogenous wound healing mechanism” involved in tissue repair through SP calling bone marrow stromal cells has been identified [19]. It was also found to stimulate concomitant EPC mobilization with bone marrow stromal cells [11]. The potential pathway for differentiation into endothelial or hepatocyte lineages is thought to be an important function of CD133+hepatic stellate cells (HSCs) during liver regeneration [20]. Expression patterns and non-polarizing cell morphology develop from CD133+HSCs in immature hepatocyte-like cells. Owing to their ability to generate hepatocytes and endothelial lineages, CD133+HSCs can directly support the repair of injured liver tissue. Therefore, it is necessary to investigate the contribution of CD133+HSCs to liver regeneration in vivo [21]. In this study, we assumed stem cell mobilization by confirming that the expression of CD133 was increased at day 2; however, this should be clarified through further studies.
PI3K comprises 85α and 110α subunits and is involved in cell growth and survival. The PI3K/Akt/mTOR pathway is an intracellular signaling pathway that is important for regulating the cell cycle. Therefore, it is directly involved in cell arrest, proliferation, cancer, and longevity. PI3K activation phosphorylates and activates Akt, localizing it to the plasma membrane [22]. The role of PI3K in liver regeneration remains unclear. PI3K activity is induced by hepatic resection. Activation of the PI3K/Akt pathway plays a critical role in the early regenerative response of the liver after resection [9]. The phosphorylation levels of mTOR, Akt, PI3K, and ERK were higher in the SP group than in the saline group. These results suggest that SP contributes to liver regeneration by regulating survival and proliferation through mTOR/Akt/PI3K and ERK phosphorylation. MAPKs (JNK, p38 MAPK, and ERK) mediate signal transduction involved in cell proliferation, differentiation, transformation, survival, and death [10]. PI3K/Akt/mTOR activation may be mainly involved in SP-induced M2 polarization; however, SP-induced ERK activation may be required for the survival and proliferation of macrophages without affecting M2 polarization [23]. SP treatment inhibits NADPH oxidase activity and reduces reactive oxygen species production. SP stimulation can block the p38 MAPK and ERK signaling pathways; the activity of phospho-p38 and phospho-ERK and the expression of NF-E2-related factor 2 decreased after SP treatment. The regulation of hyperoxic lung injury by SP through decreasing cell apoptosis, increasing antioxidant activity, and attenuating oxidative stress has been reported [24].
The limitation of this study was that in vitro experiments at the cell level were not included, resulting in unsatisfactory results for molecular mechanisms that proceeded in the short period after SP injection. To obtain accurate results on molecular mechanisms, data in minutes and hours are required through in vitro studies. Nevertheless, this study is valuable as an early preliminary study on the effect of SP on liver regeneration.
Patients and liver transplant donors with small remnant liver volume after hepatectomy are carefully selected due to concerns about poor outcome [25,26]. Our results suggesting the liver regeneration-promoting effect of SP provide the possibility to reduce the risk of liver failure, and it is expected that the range of patients eligible for hepatectomy will be expanded.
Conclusions
SP accelerated the stage of regeneration after hepatectomy in a rat model. This implies that liver function recovers earlier than in the normal regeneration process. It is expected to be helpful in clinical practice by preventing liver failure, which is caused by worsening of liver function immediately after liver resection. This effect is presumed to occur through mechanisms that promote stem cell mobilization and upregulate the MAPK/ERK pathway. However, in vitro studies are required to clarify this mechanism.
Figures
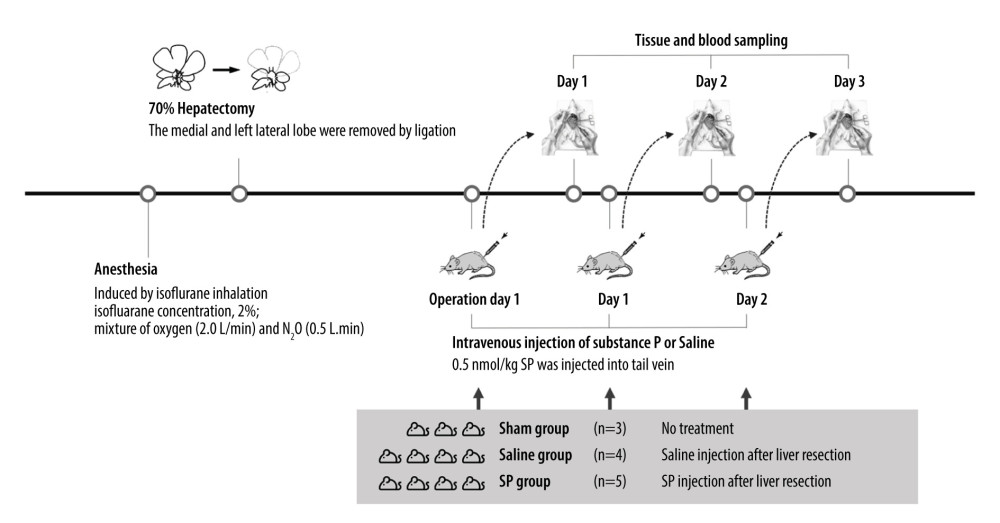 Figure 1. Experimental protocol and animal groups.
Figure 1. Experimental protocol and animal groups. 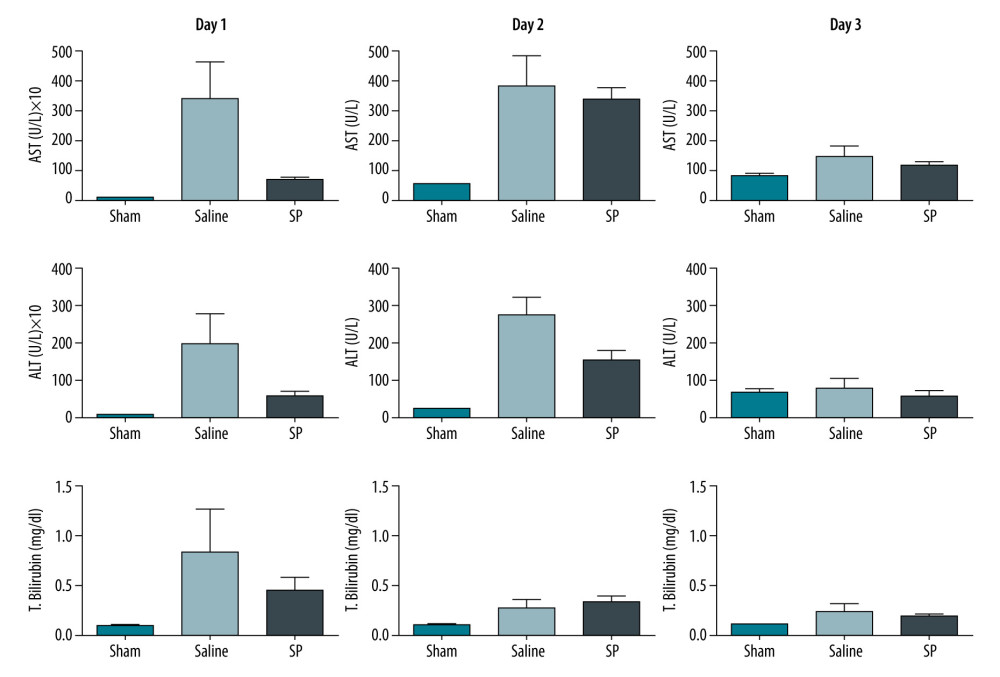 Figure 2. Liver function tests in serum were analyzed using a biochemical analyzer. AST, aspartate aminotransferase; ALT, alanine aminotransferase; and T bilirubin, total bilirubin. Prism, version 5 (GraphPad Software, Inc., San Diego, USA) was used for creation of the figure.
Figure 2. Liver function tests in serum were analyzed using a biochemical analyzer. AST, aspartate aminotransferase; ALT, alanine aminotransferase; and T bilirubin, total bilirubin. Prism, version 5 (GraphPad Software, Inc., San Diego, USA) was used for creation of the figure. 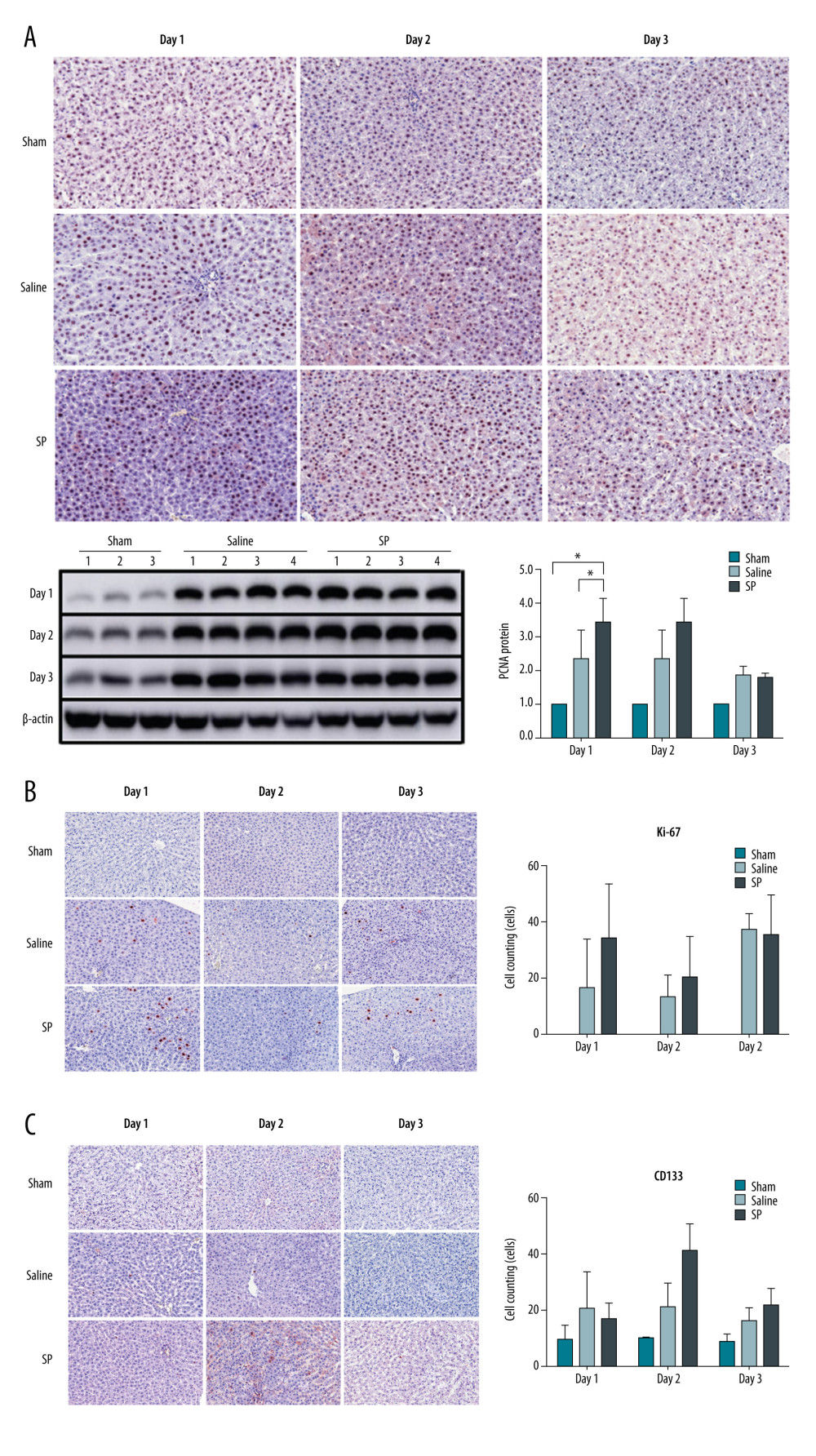 Figure 3. Immunohistochemical analysis of proliferating cell nuclear antigen (PCNA), Ki-67, and CD133 upon substance P (SP) injection after hepatectomy. After hepatectomy, SP was injected on days 1, 2, and 3. Paraffin blocks of tissues from each group were prepared by sectioning and stained using the immunohistochemistry method with PCNA (A), Ki-67 (B), and CD133 (C) antibodies. Prism, version 5 (GraphPad Software, Inc., San Diego, USA) was used for creation of the figure.
Figure 3. Immunohistochemical analysis of proliferating cell nuclear antigen (PCNA), Ki-67, and CD133 upon substance P (SP) injection after hepatectomy. After hepatectomy, SP was injected on days 1, 2, and 3. Paraffin blocks of tissues from each group were prepared by sectioning and stained using the immunohistochemistry method with PCNA (A), Ki-67 (B), and CD133 (C) antibodies. Prism, version 5 (GraphPad Software, Inc., San Diego, USA) was used for creation of the figure. 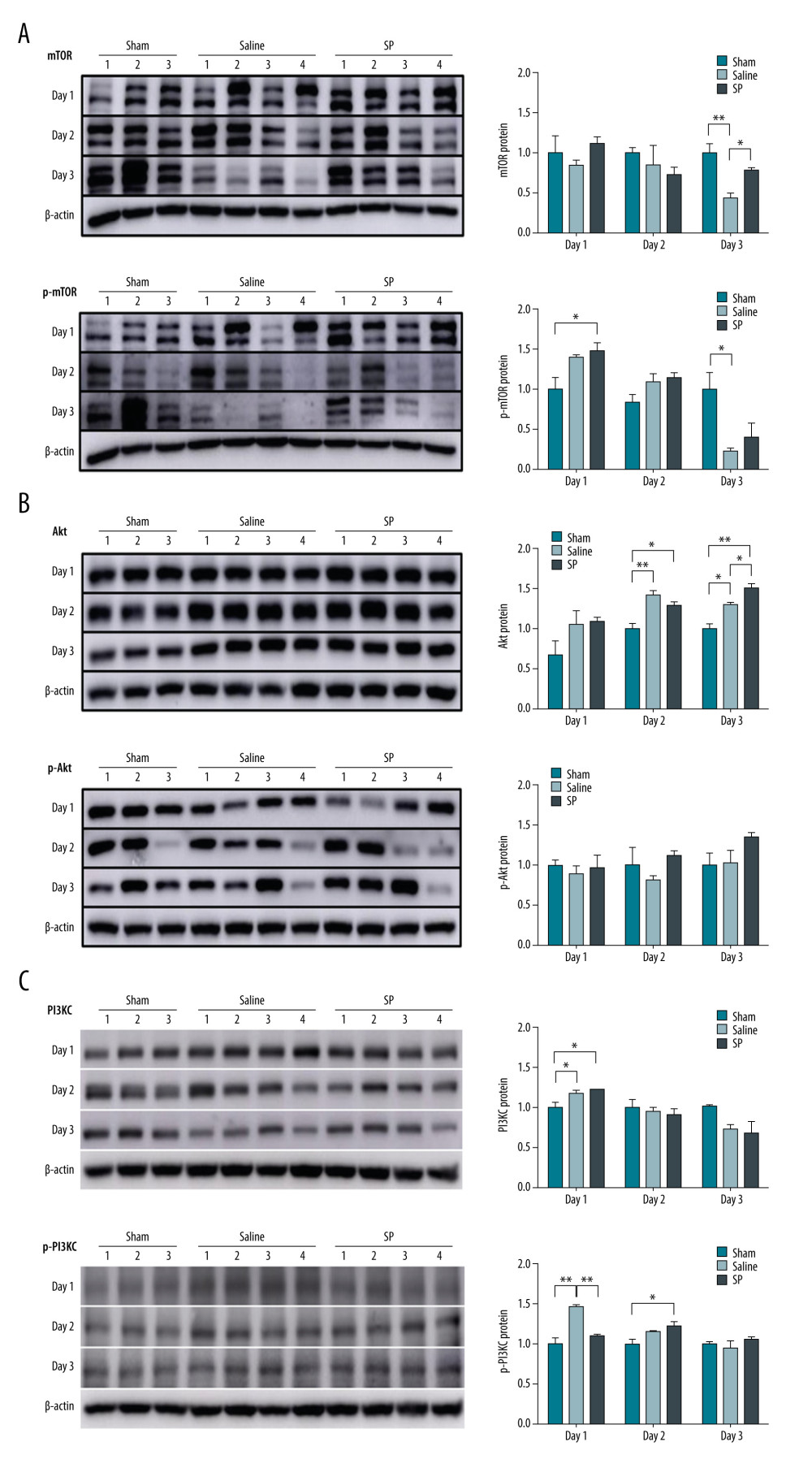 Figure 4. Expression of mTOR (A), Akt (B), and PI3KC (C) proteins. Protein expression was determined using immunoblot analysis. Tissues were lysed and 10 μg of soluble protein was separated via electrophoresis on a 10% SDS-PAGE gel. Densitometry results are presented as the relative ratios of mTOR, Akt, and PI3KC to β-actin. The data are expressed as mean±SD. * P<0.05 and ** P<0.01 comparing saline vs SP groups. Prism, version 5 (GraphPad Software, Inc., San Diego, USA) was used for creation of the figure.
Figure 4. Expression of mTOR (A), Akt (B), and PI3KC (C) proteins. Protein expression was determined using immunoblot analysis. Tissues were lysed and 10 μg of soluble protein was separated via electrophoresis on a 10% SDS-PAGE gel. Densitometry results are presented as the relative ratios of mTOR, Akt, and PI3KC to β-actin. The data are expressed as mean±SD. * P<0.05 and ** P<0.01 comparing saline vs SP groups. Prism, version 5 (GraphPad Software, Inc., San Diego, USA) was used for creation of the figure. 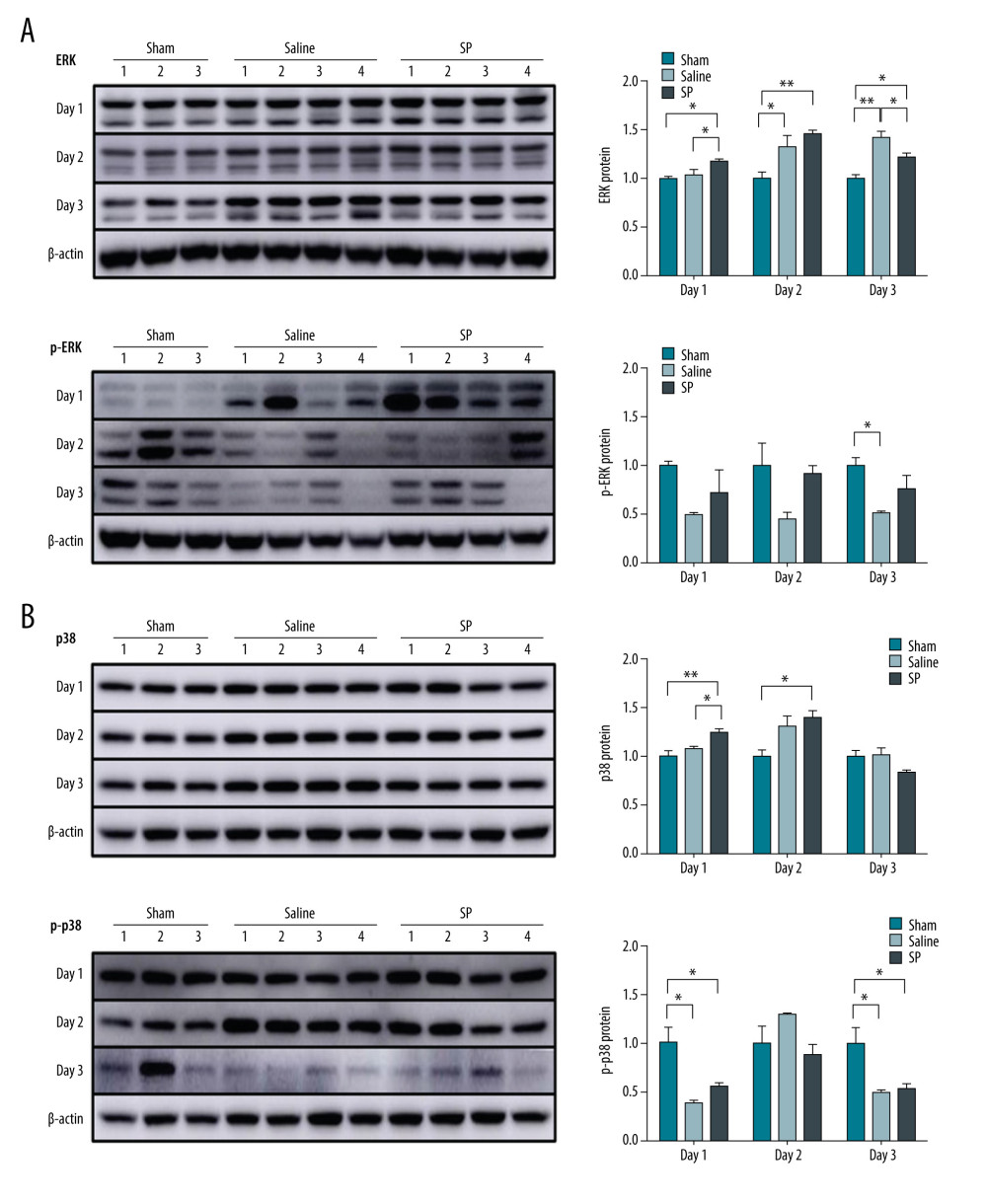 Figure 5. Expression of ERK (A) and p38 (B) protein. Protein expression was determined using immunoblot analysis. Tissues were lysed and 10 μg of soluble protein was separated via electrophoresis on a 10% SDS-PAGE gel. Densitometry results are presented as the relative ratios of ERK and p38 to β-actin. The data are expressed as mean±SD. * P<0.05 and ** P<0.01 comparing saline vs SP groups. Prism, version 5 (GraphPad Software, Inc., San Diego, USA) was used for creation of the figure.
Figure 5. Expression of ERK (A) and p38 (B) protein. Protein expression was determined using immunoblot analysis. Tissues were lysed and 10 μg of soluble protein was separated via electrophoresis on a 10% SDS-PAGE gel. Densitometry results are presented as the relative ratios of ERK and p38 to β-actin. The data are expressed as mean±SD. * P<0.05 and ** P<0.01 comparing saline vs SP groups. Prism, version 5 (GraphPad Software, Inc., San Diego, USA) was used for creation of the figure. References
1. Kwon YJ, Lee KG, Choi D, Clinical implications of advances in liver regeneration: Clin Mol Hepatol, 2015; 21; 7-13
2. Pomfret EA, Pomposelli JJ, Jenkins RL, Live donor liver transplantation: J Hepatol, 2001; 34; 613-24
3. De Felipe C, Herrero JF, O’Brien JA, Altered nociception, analgesia and aggression in mice lacking the receptor for substance P: Nature, 1998; 392; 394-97
4. Muñoz M, Coveñas R, Involvement of substance P and the NK-1 receptor in human pathology: Amino acids, 2014; 46; 1727-50
5. Steinhoff MS, von Mentzer B, Geppetti P, Tachykinins and their receptors: Contributions to physiological control and the mechanisms of disease: Physiol Rev, 2014; 94; 265-301
6. Wan Y, Meng F, Wu N, Substance P increases liver fibrosis by differential changes in senescence of cholangiocytes and hepatic stellate cells: Hepatology, 2017; 66; 528-41
7. Suvas S, Role of substance P neuropeptide in inflammation, wound healing, and tissue homeostasis: J Immunol, 2017; 199; 1543-52
8. Katsanos GS, Anogeianaki A, Orso C, Impact of substance P on cellular immunity: J Biol Regul Homeost Agents, 2008; 22; 93-98
9. Jackson LN, Larson SD, Silva SR, PI3K/Akt activation is critical for early hepatic regeneration after partial hepatectomy: Am J Physiol Gastrointest Liver Physiol, 2008; 294; G1401-10
10. Dhillon AS, Hagan S, Rath O, Kolch W, MAP kinase signalling pathways in cancer: Oncogene, 2007; 26; 3279-90
11. Hong HS, Lee J, Lee E, A new role of substance P as an injury-inducible messenger for mobilization of CD29(+) stromal-like cells: Nat Med, 2009; 15; 425-35
12. Michalopoulos GK, Principles of liver regeneration and growth homeostasis: Compr Physiol, 2013; 3; 485-513
13. Nakamura RM, Miyada DS, Moyer DL, Effect of liver regeneration following partial hepatectomy on the uptake of tritiated thymidine in the pituitary gland of the rat: Nature, 1963; 199; 707-8
14. Michalopoulos GK, Liver regeneration: J Cell Physiol, 2007; 213; 286-300
15. Ojanguren I, Ariza A, Llatjós M, Proliferating cell nuclear antigen expression in normal, regenerative, and neoplastic liver: A fine-needle aspiration cytology and biopsy study: Hum Pathol, 1993; 24; 905-8
16. Theocharis SE, Skopelitou AS, Margeli AP, Proliferating cell nuclear antigen (PCNA) expression in regenerating rat liver after partial hepatectomy: Dig Dis Sci, 1994; 39; 245-52
17. Assy N, Gong Y, Zhang M, Use of proliferating cell nuclear antigen as a marker of liver regeneration after partial hepatectomy in rats: J Lab Clin Med, 1998; 131; 251-56
18. Forbes SJ, Newsome PN, Liver regeneration – mechanisms and models to clinical application: Nat Rev Gastroenterol Hepatol, 2016; 13; 473-85
19. Hong HS, Kim DY, Yoon KJ, Son Y, A new paradigm for stem cell therapy: substance-P as a stem cell-stimulating agent: Arch Pharm Res, 2011; 34; 2003-6
20. Kordes C, Sawitza I, Müller-Marbach A, CD133+ hepatic stellate cells are progenitor cells: Biochem Biophys Res Commun, 2007; 352; 410-17
21. Taniguchi E, Kin M, Torimura T, Endothelial progenitor cell transplantation improves the survival following liver injury in mice: Gastroenterology, 2006; 130; 521-31
22. King D, Yeomanson D, Bryant HE, PI3King the lock: Targeting the PI3K/Akt/mTOR pathway as a novel therapeutic strategy in neuroblastoma: J Pediatr Hematol Oncol, 2015; 37; 245-51
23. Lim JE, Chung E, Son Y, A neuropeptide, Substance-P, directly induces tissue-repairing M2 like macrophages by activating the PI3K/Akt/mTOR pathway even in the presence of IFNγ: Sci Rep, 2017; 7; 9417
24. Huang B, Li Q, Xu S, Substance P protects against hyperoxic-induced lung injury in neonatal rats: Exp Lung Res, 2015; 41; 12-20
25. Hirashita T, Ohta M, Iwashita Y, Risk factors of liver failure after right-sided hepatectomy: Am J Surg, 2013; 206(3); 374-79
26. Walter J, Burdelski M, Bröring DC, Chances and risks in living donor liver transplantation: Dtsch Arztebl Int, 2008; 105(6); 101-7
Figures
 Figure 1. Experimental protocol and animal groups.
Figure 1. Experimental protocol and animal groups. Figure 2. Liver function tests in serum were analyzed using a biochemical analyzer. AST, aspartate aminotransferase; ALT, alanine aminotransferase; and T bilirubin, total bilirubin. Prism, version 5 (GraphPad Software, Inc., San Diego, USA) was used for creation of the figure.
Figure 2. Liver function tests in serum were analyzed using a biochemical analyzer. AST, aspartate aminotransferase; ALT, alanine aminotransferase; and T bilirubin, total bilirubin. Prism, version 5 (GraphPad Software, Inc., San Diego, USA) was used for creation of the figure. Figure 3. Immunohistochemical analysis of proliferating cell nuclear antigen (PCNA), Ki-67, and CD133 upon substance P (SP) injection after hepatectomy. After hepatectomy, SP was injected on days 1, 2, and 3. Paraffin blocks of tissues from each group were prepared by sectioning and stained using the immunohistochemistry method with PCNA (A), Ki-67 (B), and CD133 (C) antibodies. Prism, version 5 (GraphPad Software, Inc., San Diego, USA) was used for creation of the figure.
Figure 3. Immunohistochemical analysis of proliferating cell nuclear antigen (PCNA), Ki-67, and CD133 upon substance P (SP) injection after hepatectomy. After hepatectomy, SP was injected on days 1, 2, and 3. Paraffin blocks of tissues from each group were prepared by sectioning and stained using the immunohistochemistry method with PCNA (A), Ki-67 (B), and CD133 (C) antibodies. Prism, version 5 (GraphPad Software, Inc., San Diego, USA) was used for creation of the figure. Figure 4. Expression of mTOR (A), Akt (B), and PI3KC (C) proteins. Protein expression was determined using immunoblot analysis. Tissues were lysed and 10 μg of soluble protein was separated via electrophoresis on a 10% SDS-PAGE gel. Densitometry results are presented as the relative ratios of mTOR, Akt, and PI3KC to β-actin. The data are expressed as mean±SD. * P<0.05 and ** P<0.01 comparing saline vs SP groups. Prism, version 5 (GraphPad Software, Inc., San Diego, USA) was used for creation of the figure.
Figure 4. Expression of mTOR (A), Akt (B), and PI3KC (C) proteins. Protein expression was determined using immunoblot analysis. Tissues were lysed and 10 μg of soluble protein was separated via electrophoresis on a 10% SDS-PAGE gel. Densitometry results are presented as the relative ratios of mTOR, Akt, and PI3KC to β-actin. The data are expressed as mean±SD. * P<0.05 and ** P<0.01 comparing saline vs SP groups. Prism, version 5 (GraphPad Software, Inc., San Diego, USA) was used for creation of the figure. Figure 5. Expression of ERK (A) and p38 (B) protein. Protein expression was determined using immunoblot analysis. Tissues were lysed and 10 μg of soluble protein was separated via electrophoresis on a 10% SDS-PAGE gel. Densitometry results are presented as the relative ratios of ERK and p38 to β-actin. The data are expressed as mean±SD. * P<0.05 and ** P<0.01 comparing saline vs SP groups. Prism, version 5 (GraphPad Software, Inc., San Diego, USA) was used for creation of the figure.
Figure 5. Expression of ERK (A) and p38 (B) protein. Protein expression was determined using immunoblot analysis. Tissues were lysed and 10 μg of soluble protein was separated via electrophoresis on a 10% SDS-PAGE gel. Densitometry results are presented as the relative ratios of ERK and p38 to β-actin. The data are expressed as mean±SD. * P<0.05 and ** P<0.01 comparing saline vs SP groups. Prism, version 5 (GraphPad Software, Inc., San Diego, USA) was used for creation of the figure. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








