19 January 2022: Original Paper
Efficacy of Nitric Oxide-Releasing Nanofibers in Reducing Renal Ischemia-Reperfusion Injury in a Rat Model
Hyunmin KoDOI: 10.12659/AOT.934800
Ann Transplant 2022; 27:e934800
Abstract
BACKGROUND: This study aimed to analyze the preventive effect of nitric oxide (NO)-releasing nanofibers against ischemia-reperfusion injury (IRI) and to determine the mechanism of action as a novel NO delivery system in a rat model.
MATERIAL AND METHODS: Eight-week-old male Sprague-Dawley rats, weighing 250 to 280 g, were divided into 3 groups: sham, untreated (n=5); control, renal ischemia injury for 55 min (n=4); and NO24, renal ischemia injury for 55 min with kidney wrapping of NO-releasing nanofiber for 24 h (n=6). mRNA expression was measured by real-time polymerase chain reaction (PCR), whereas protein expression was assessed by immunohistochemistry and western blot analysis.
RESULTS: Serum creatinine levels in the sham, control, and NO24 groups were 0.48±0.08, 4.66±0.33, and 2.60±1.00 mg/dL, respectively (P=0.002). Anti-apoptotic Bcl-2 protein expression differed significantly between the control and the NO24 groups (Bcl-2/β-actin; control, 0.50±0.12; NO24, 1.56±0.56; P=0.024). mRNA expression level of the inflammatory cytokine tumor necrosis factor-α (TNF-α) was significantly higher in the control group (23.24±11.32, P=0.016) than in the sham group (1.00±1.21), and mRNA expression of TNF-α in the NO24 group (1.28±1.16, P=0.010) was significantly lower than in the control group. Histological analysis revealed decreased atrophy and necrosis in the NO24 group compared to those in the control group.
CONCLUSIONS: This study demonstrated that kidney wrapping of NO-releasing nanofibers had a protective effect against kidney IRI through anti-apoptotic and anti-inflammatory mechanisms.
Keywords: Kidney Transplantation, Nanofibers, Nitric Oxide, Animals, Kidney, Male, Rats, Rats, Sprague-Dawley
Background
Several clinical series have identified ischemia-reperfusion injury (IRI) as a major problem and a critical factor influencing the outcome of transplantation in patients [1,2]. IRI is also common after abdominal aortic aneurysm repair and is related to patient prognosis [3]. Microvascular injury is associated with IRI. After reperfusion, endothelial cells are activated, resulting in the production of high levels of reactive oxygen species (ROS) and low levels of nitric oxide (NO); this imbalance leads to an inflammatory response [4]. As an inflammatory response, leukocytes are recruited, and free radicals and inflammatory cytokines are released. ROS damage cellular proteins, DNA, and plasma membranes, resulting in the release of more free radicals. In addition, ROS increase apoptosis by participating in the apoptosis pathway [4]. Although the pathophysiology of kidney IRI is poorly understood, early pathologic changes in the kidney after IRI include vasoconstriction, endothelial cell activation, and tubular edema. Reperfusion increases endothelial expression of adhesion molecules, leads to leukocyte recruitment and activation, increases production of ROS, and promotes inflammatory responses [5,6].
Studies focused on reducing IRI have been conducted in various fields, such as organ transplantation, cerebrovascular disease, and wound healing [7,8]. During organ transplantation, warm ischemia leads to activation of various inflammatory pathways culminating in cellular injury to the organ [9]. Warm IRI can be divided into early and late phases. The early phase occurs within the first 2 h after reperfusion and the late phase occurs within 6 to 48 h after reperfusion. Cytokine and chemokine production occurs during the early phase of IRI. Tumor necrosis factor-alpha (TNF-α) and interleukin (IL) significantly increase early and systemically in the serum within minutes after reperfusion. These cytokines upregulate the production of other cytokines, chemokines, and adhesion molecules, which are all critical to the pattern of injury observed in the late phase of IRI [10].
The gases NO, CO, and H2S have been recognized as important signaling molecules against IRI. They regulate vascular tone and ameliorate inflammatory effects [8]. Particularly, NO has been demonstrated to play a key role in repairing tissue damaged by IRI [11], and previous studies have reported a protective effect of NO against IRI. NO mediates anti-inflammatory responses through reduction of neutrophil infiltration and reduction of proinflammatory cytokines in IRI conditions [12]. NO suppresses apoptosis by downregulating the expression of p53 gene, which promotes apoptosis. After IRI, the p53 gene expression level is increased. However, when an exogenous NO donor was given during IRI, downregulation of p53 was correlated with decreased apoptosis. Furthermore, it has been demonstrated that NO inhibits the activity of the caspase cascade, which plays an important role in the initiation of apoptosis [13].
Intra-renal administration of the NO-releasing molecule molsidomine [14], injection of L-arginine [15], and use of the NO-releasing nonsteroidal anti-inflammatory drugs (NO-NSAIDs) furoxan and zeolite [16] have been tested as methods to supply NO. However, NO administered via these routes can act systemically; therefore, it is important to develop a method that minimizes the action of NO on non-target organs and effectively provides NO to target organs. Previous studies reported that the use of high NO-loading hydrogels [17] or NO-supplying polymers [18] results in local diffusion of NO, reducing its loss due to release into the bloodstream; however, the application of these approaches is limited to vascular grafts or stents. Echogenic liposomes loaded with gaseous NO [19] have been studied for site-specific NO delivery. Although liposomes prevent gaseous NO scavenging by hemoglobin, their efficacy is poor because of continuous blood flow. Thus, there are still many challenges to be overcome for realizing the clinical application of these methods.
To overcome these limitations, NO-releasing nanofibers were used to supply NO directly to the target organ. The aim of this study was to analyze the preventive effect of NO-releasing nanofiber against IRI and elucidate its mechanism of action as a novel NO delivery system in a rat model.
Material and Methods
ANIMALS:
Healthy male Sprague-Dawley rats (8 weeks old, body weight 250–280 g; Young Bio Co, Seoul, Korea) were housed in standard animal laboratories under a controlled temperature (23±2°C) with a 12-h light-dark cycle and ad libitum access to tap water and standard laboratory chow until 12 h before the experiment, when the animals were fasted. All animals were maintained in accordance with the recommendations of the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals of the Korean Academy of Medical Sciences. The experiments were approved by the Institutional Animal Care and Use Committee of Kyung Hee University.
SYNTHESIS OF NO-RELEASING NANOFIBERS:
Since the synthesis process and materials of NO-releasing nanofibers have been previously described in our literature [20], the details were omitted here (Supplementary Figure 1).
EXPERIMENTAL PROTOCOLS:
Fifteen rats were divided into 3 groups: sham, untreated group (n=5); control, renal ischemia injury for 55 min (n=4); and NO24, renal ischemia injury for 55 min with the kidney wrapped with NO nanofiber for 24 h (n=6). All procedures were performed with the rats under anesthesia induced by isoflurane inhalation (isoflurane concentration, 1.5–3%; oxygen flow 0.5 L/min). One week before the experiment, all rats underwent right nephrectomy.
In the sham group (n=5), rats were anesthetized and submitted to the complete surgical procedure without aortic clamping. First, in the NO24 group, the kidney was wrapped with an NO sheet, and 1 h later, the control and NO24 groups were started with ischemic injury. The ischemia-reperfusion procedure was performed by making a left lateral incision and then the renal artery and vein were clamped for 55 min using a microvascular clamp. After 24 h, the sheet was removed from the kidney in the NO24 group. After 48 h, tissue and blood samples were obtained (Supplementary Figure 2).
DETECTION OF THE LEVELS OF CREATININE IN THE SERUM:
Serum was obtained through the intra-cardiac puncture at 48 h after the operation. Creatinine levels in the serum were determined using a biochemical analyzer at the Korean Animal Medical Science Institute.
WESTERN BLOT ANALYSIS:
Tissues were homogenized and then underwent lysis using lysis buffer containing 1 mM PMSF (Cell Signaling Technology, Boston, MA, USA). The protein concentration was determined using a BCA protein assay (Thermo Fisher Scientific, Rockford, IL, USA), according to the manufacturer’s protocol. Ten micrograms of protein were fractionated using 8–12% sodium dodecyl-sulfate polyacrylamide gel electrophoresis and transferred via electrophoresis to nitrocellulose blotting membranes. The membranes were blocked with 1% bovine serum albumin for 1 h at room temperature and then incubated overnight at 4°C with antibodies against Bax, Bcl-2, NF-κB, cleaved caspase-3, and β-actin (Cell Signaling Technology), which were diluted 1: 1000 with Tris-buffered saline containing 0.05% Tween-20 (TBS-T). After washing with TBS-T for 1 h, the membranes were incubated for 1 h at room temperature with anti-rabbit and anti-mouse horseradish peroxidase-conjugated secondary antibodies diluted 1: 2500 in TBS-T. The membranes were subsequently washed with TBS-T for 1 h, and proteins were developed using Amersham ECL Prime reagent (GE Healthcare Life Sciences, UK). The protein band densities were quantified using Image J software (NIH, USA).
RNA EXTRACTION AND REAL-TIME POLYMERASE CHAIN REACTION:
Total RNA was extracted from the rat whole kidneys using TRIzol (Invitrogen, Carlsbad, CA, USA). First-strand cDNA synthesis was performed with 2 μg of total RNA and transcribed to cDNA using a reverse transcription system with Oligo(dT)18 (Clontech Laboratory, A Takara Bio Company, CA, USA) in a 20-μL reaction volume. Real-time polymerase reaction chain reaction (PCR) was performed using the StepOnePlus Real-Time PCR system (Applied Biosystems, USA). Amplification products were analyzed by a melting curve, which confirmed the presence of a single PCR product in all reactions. Levels of messenger RNA (mRNA) were normalized to those of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primer sequences were as follows:
The amplification program was 95°C for 3 min and then 40 cycles consisting of 95°C for 10 s, 62°C for 10 s, and 72°C for 10 s. The crossing point of target genes with GAPDH was calculated using the formula 2−(target gene–GAPDH), and the relative amounts were quantified.
ANALYSIS OF RENAL MORPHOLOGY:
Light microscopy was performed for the analysis of renal morphology. For light microscopy, kidney tissue was fixed in 10% neutral buffered formalin, embedded in paraffin, cut into 3-μm sections, and stained with hematoxylin and eosin and periodic acid-Schiff. Semi-quantitative scoring of tubular damage was performed by quantification of the IRI standard with the following categories: grade 0, normal; grade 1, tubular damage with tubular necrosis in 1% to 25%; grade 2, tubular damage with tubular necrosis in 26% to 50%; grade 3, severe tubular damage with tubular necrosis in 51% to 75% of tubules, without the presence of partial occlusion of the tubular lumen by cellular debris; grade 4, severe tubular damage with tubular necrosis in 76% to 99% of tubules, without the presence of partial occlusion of the tubular lumen by cellular debris; and grade 5, severe tubular damage with tubular necrosis in 100% of tubules, without the presence of partial occlusion of the tubular lumen by cellular debris. One hundred glomeruli in each group were examined using the previously described semi-quantitative scale.
STATISTICAL ANALYSIS:
Data are expressed as mean±standard deviation (SD) and these were compared statistically using the Kruskal-Wallis and Mann-Whitney U tests. All statistical analyses were performed using SPSS statistical software (IBM Corp, Armonk, NY, USA) and GraphPad Prism (GraphPad Software, Inc, San Diego, USA). Values of
Results
SERUM CREATININE LEVELS:
Serum creatinine levels were elevated at 48 h after surgery in all animals that had undergone ischemia-reperfusion. This elevation was blunted in the NO24 group compared with in the control group. Creatinine levels in the sham, control, and NO24 groups were 0.48±0.08, 4.66±0.33, and 2.60±1.00 mg/dL, respectively (P=0.002). Serum creatinine levels in the NO24 group were significantly lower than those in the control group (P=0.010) (Figure 1).
EXPRESSION OF BAX, BCL-2, CLEAVED CASPASE-3, AND NF-κB:
Western blot analysis of tissues obtained from the renal cortices of animals in each group revealed that the expression of Bcl-2 protein was significantly different between the control and NO24 groups (Bcl-2/β-actin; control, 0.50±0.12; NO24, 1.56±0.56; P=0.024). Compared with the sham group, Bax protein expression was increased by more than 4-fold in the control group but was only slightly increased in the NO24 group (Bax/β-actin; sham, 0.27±0.14; control, 1.17±0.30; NO24, 0.53±0.38; P=0.041). Expression of cleaved caspase-3 also exhibited significant differences among groups (sham, 0.25±0.06; control, 0.81±0.11; NO24, 0.60±0.29; P=0.043). NF-κB exhibited similar patterns, with no significant differences between groups (sham, 0.20±0.03; control, 0.35±0.10; NO24, 0.28±0.12; P=0.076) (Figure 2A).
In the renal medulla, there was no significant difference in the expression levels of Bax, Bcl-2, cleaved caspase-3, and NF-κB among the sham, control, and NO24 groups (P=0.142, P=0.748, P=0.254, and P=0.063, respectively) (Figure 2B).
MRNA EXPRESSION OF IL-1β, IL-6, IL-9, TNF-α, ICAM, AND VCAM:
Real-time PCR data were expressed relative to the mean expression in the sham group, which was indicated as 1.00. mRNA expression of the inflammation-related cytokine TNF-α in the renal cortex was significantly upregulated in the control group (23.24±11.32, P=0.016) compared with in the sham group (1.00±1.21). In contrast, the mRNA expression of TNF-α in the NO24 group (1.28±1.16, P=0.010) was significantly lower than in the control group. Moreover, IL-9 mRNA expression differed significantly among groups (sham, 1.00±0.93; control, 4.61±2.87; and NO24, 2.71±1.74; P=0.038). However, IL-1β and IL-6 exhibited similar mRNA expression levels, without significant differences (P=0.324 and P=0.111, respectively) (Figure 3A).
In the medulla, only IL-6 mRNA expression was significantly lower in the NO24 group (0.65±0.42) compared with that in the control group (4.05±2.15, P=0.010). However, the mRNA expression levels of other inflammatory cytokines did not differ significantly between the control and NO24 groups (IL-1β, P=0.476; IL-9, P=0.914; TNF-α, P=0.114) (Figure 3B).
The mRNA expression of ICAM and VCAM were not significantly different among groups in either the renal cortex or the medulla (Figure 3A, 3B).
HISTOLOGICAL CHANGES:
The periodic acid-Schiff scores of renal morphology in the sham, control, and NO24 groups were not significantly different. The brush border of the proximal tubule was depleted in the control and NO24 groups compared with that in the sham group. The NO24 group showed less atrophy and necrosis than the control group. Interestingly, the control group exhibited many hyaline casts (Figure 4A–4D).
Discussion
This study demonstrated that wrapping of the kidney with NO-releasing nanofiber reduced the aggravation of renal function due to IRI, as evidenced by blunted increases in serum creatinine levels. Histological changes indicated decreased tubular necrosis and atrophy in the NO24 group compared with the control group, suggesting that NO-releasing nanofibers had a protective effect against IRI.
NO-supplying materials have been previously investigated. Rodriguez-Pena et al [14] showed that intra-renal administration of molsidomine before reperfusion improved renal function and decreased inflammatory responses after IRI. Barakat et al [15] demonstrated that IRI impaired graft function during the first week after transplantation, and that injection with L-arginine before ischemia antagonized graft deterioration and improved the morphological appearance. Zhu et al [16] showed that indomethacin had a protective effect in a certain dose range; notably, its effect on IRI in mice was related to COX-1/2 blockade.
We applied a recently developed NO-releasing nanofiber in this experiment to supply NO. A limitation of previous methods was that the molecules capable of supplying NO were delivered by intravenous administration, which can lead to unpredictable systemic effects. However, NO-releasing nanofibers have the advantage of minimizing systemic effects because they are delivered directly to the target tissue through diffusion. In this study, the best outcomes were obtained when the NO sheet was wrapped 1 h before ischemia and then removed after 24 h. Regarding the physiological properties of NO-releasing nanofibers, the time to reach half of the maximum concentration was consistently 5 min, the maximum dose was approached within 1 h, and the duration to reach the maximum dose was 25 h [21].
To compare kidney function, serum creatinine was measured 48 h after surgery. In the control group with kidney IRI, kidney function was significantly worse than that in the sham group without IRI. In the NO24 group, kidney function was significantly improved compared with that of the control group. In analysis of the degree of damage through semi-quantitative scoring of tubular damage, in the NO24 group, the damage including atrophy and necrosis was 60%, which was found to reduce tubular injury, compared with the value of 71% in the control group. These findings demonstrate the protective effect of NO-releasing nanofibers on IRI.
To understand the mechanisms underlying the protective effect of NO-releasing nanofibers against IRI, we first analyzed apoptosis-regulatory proteins. NO exhibits pro-apoptotic and anti-apoptotic effects [13,22]. A previous study suggested that the Bax/Bcl-2 ratio regulates caspase-3 expression and modulates apoptosis [23]. Cleaved caspase-3 plays an important role in the caspase cascade of the apoptotic pathway; the activation of caspase-3 leads to DNA fragmentation and cell death in the final stage of apoptosis [24]. In the present study, we found that Bax protein was downregulated, whereas Bcl-2 protein was upregulated in the renal cortex in the NO24 group compared with in the control group. In addition, the protein expression levels of cleaved caspase-3 and NF-κB were decreased in the NO24 group. These results suggest that NO absorbed from nanofibers wrapped around the kidney protected against renal damage due to IRI by modulating apoptosis-related genes and inhibiting NF-κB expression (Supplementary Figure 3).
In IRI, the cytokine TNF-α functions to stimulate neutrophil infiltration [25], induce the production of other inflammatory cytokines [14], and activate NF-κB, a nuclear protein that regulates inflammatory responses [26]. Previous studies demonstrated that NO inhibits TNF-α and IL-1 [14,25,27]. In the present study, the mRNA expression of TNF-α was significantly upregulated in the control group compared with other groups, while the NO24 and sham groups expressed similar TNF-α mRNA levels. The inflammatory cytokines IL-1β, IL-6, and IL-9 mRNA also showed similar trends. Thus, our results are consistent with those of previous studies and suggest that NO absorbed from nanofiber wrapping the kidney protects against renal damage from IRI through the inhibition of inflammation-related genes. After IRI, cell adhesion molecules (CAMs) are upregulated by inflammatory cytokines [28]. CAMs mediate the initial attachment between polymorphonuclear neutrophils and activated endothelium [29]. NO inhibits TNF-α and IL-1β and leads to downregulation of VCAM, ICAM, and endothelial-leukocyte adhesion molecule-1 [30]. However, the results of the present study did not support previous reports in this context. In other words, there were no significant differences in ICAM and VCAM levels between the control and NO24 groups.
In this study, the renal cortex and medulla were examined separately. Because NO from NO-releasing nanofibers was transferred to tissues by diffusion, it was assumed that the amounts of NO reaching the cortex and medulla would differ depending on the manner of wrapping the NO sheet around the kidney. This hypothesis was supported by the results of this study. The NO-releasing nanofibers showed an excellent effect in the renal cortex but not in the medulla. This is a limitation of kidney wrapping with NO-releasing nanofibers, and further studies are needed to optimize their efficacy through the control of release rate, concentration, and wrapping time of nanofiber sheets. In the design of this experiment, another control group, kidney wrapped nanofiber without NO, was not included, so the robustness of the study results may be lacking. In addition, further experiments are needed to verify the concentration of NO and to prove the protective mechanism of NO against IRI.
Conclusions
This study demonstrated the protective effects of kidney wrapping with NO-releasing nanofibers against IRI through anti-apoptotic and anti-inflammatory activities. Our novel NO delivery approach may be valuable for decreasing damage due to renal IRI in various surgical settings.
Figures
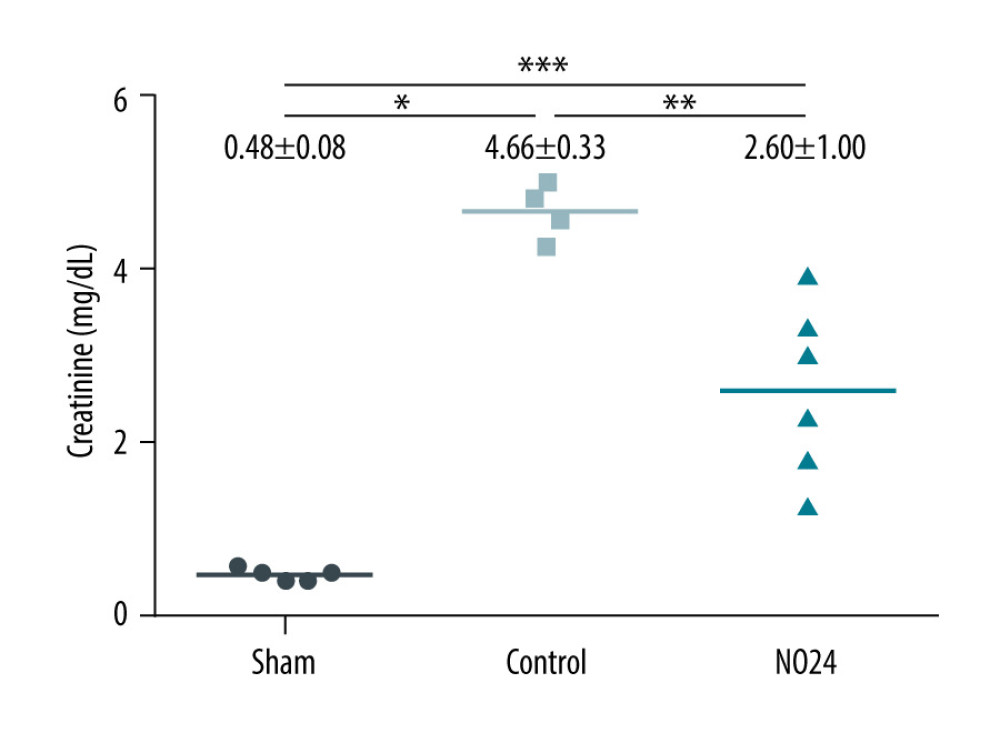 Figure 1. Serum levels of creatinineSerum creatinine levels were analyzed using a biochemical analyzer at 48 h after surgery. Serum creatinine level increased at 48 h after surgery in all animals that had undergone ischemia-reperfusion. However, it was significantly lower in the NO24 group than in the control group. * P<0.05, sham group vs control group; ** P<0.05, control group vs NO24 group; and *** P<0.05, sham group vs control group vs NO24 group were considered as significant. Prism, version 5 (GraphPad Software, Inc, San Diego, CA, USA) was used to create the figure.
Figure 1. Serum levels of creatinineSerum creatinine levels were analyzed using a biochemical analyzer at 48 h after surgery. Serum creatinine level increased at 48 h after surgery in all animals that had undergone ischemia-reperfusion. However, it was significantly lower in the NO24 group than in the control group. * P<0.05, sham group vs control group; ** P<0.05, control group vs NO24 group; and *** P<0.05, sham group vs control group vs NO24 group were considered as significant. Prism, version 5 (GraphPad Software, Inc, San Diego, CA, USA) was used to create the figure. 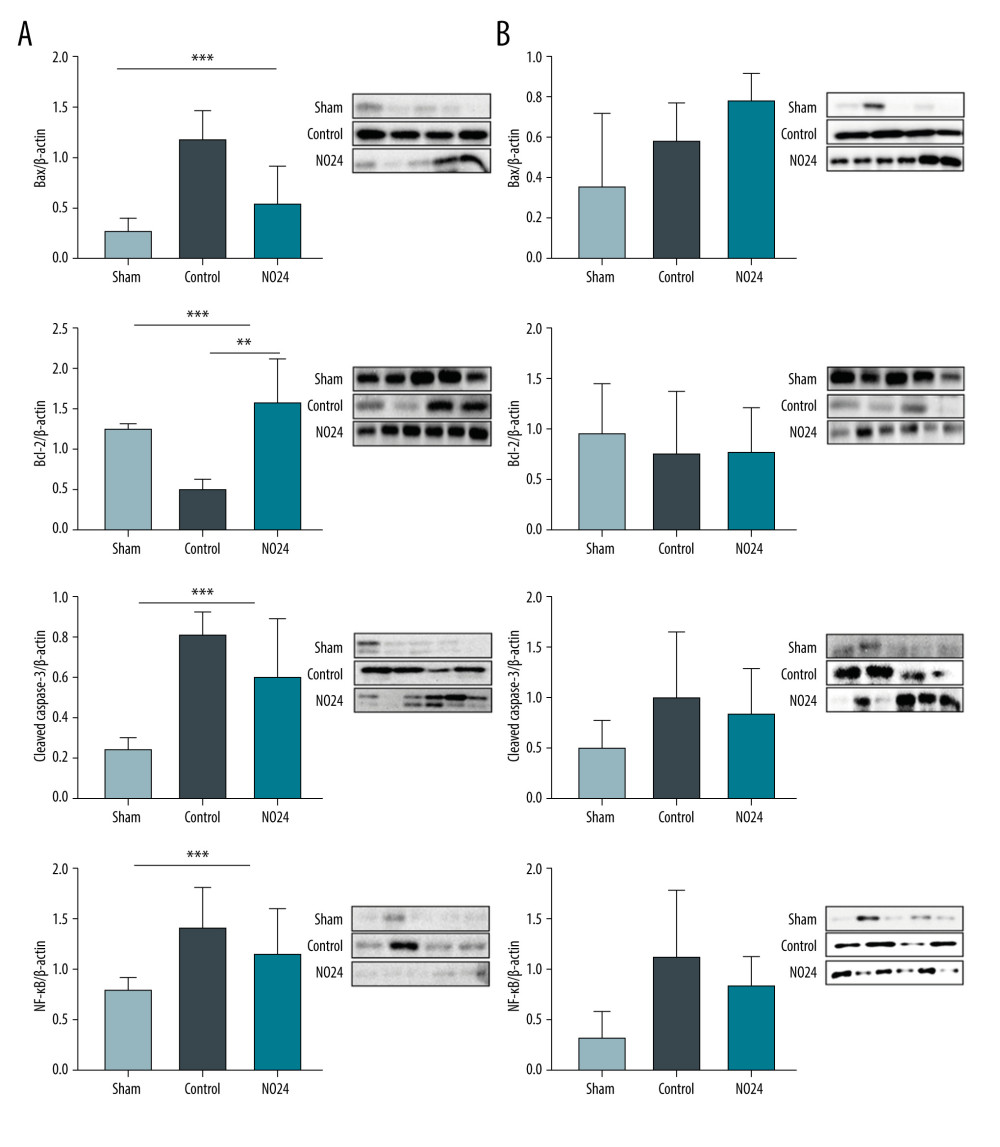 Figure 2. Expression of apoptosis- and nuclear-related protein in renal cortex and medullamRNA expression was determined by immunoblot analysis. (A) Bax, Bcl-2, cleaved caspase-3, and nuclear protein NF-κB activities in the renal cortex were determined but (B) showed no difference in the medulla. * P<0.05, sham group vs control group; ** P<0.05, control group vs NO24 group; and *** P<0.05, sham group vs control group vs NO24 group were considered as significant. Prism, version 5 (GraphPad Software, Inc, San Diego, CA USA) was used to create the figure.
Figure 2. Expression of apoptosis- and nuclear-related protein in renal cortex and medullamRNA expression was determined by immunoblot analysis. (A) Bax, Bcl-2, cleaved caspase-3, and nuclear protein NF-κB activities in the renal cortex were determined but (B) showed no difference in the medulla. * P<0.05, sham group vs control group; ** P<0.05, control group vs NO24 group; and *** P<0.05, sham group vs control group vs NO24 group were considered as significant. Prism, version 5 (GraphPad Software, Inc, San Diego, CA USA) was used to create the figure. 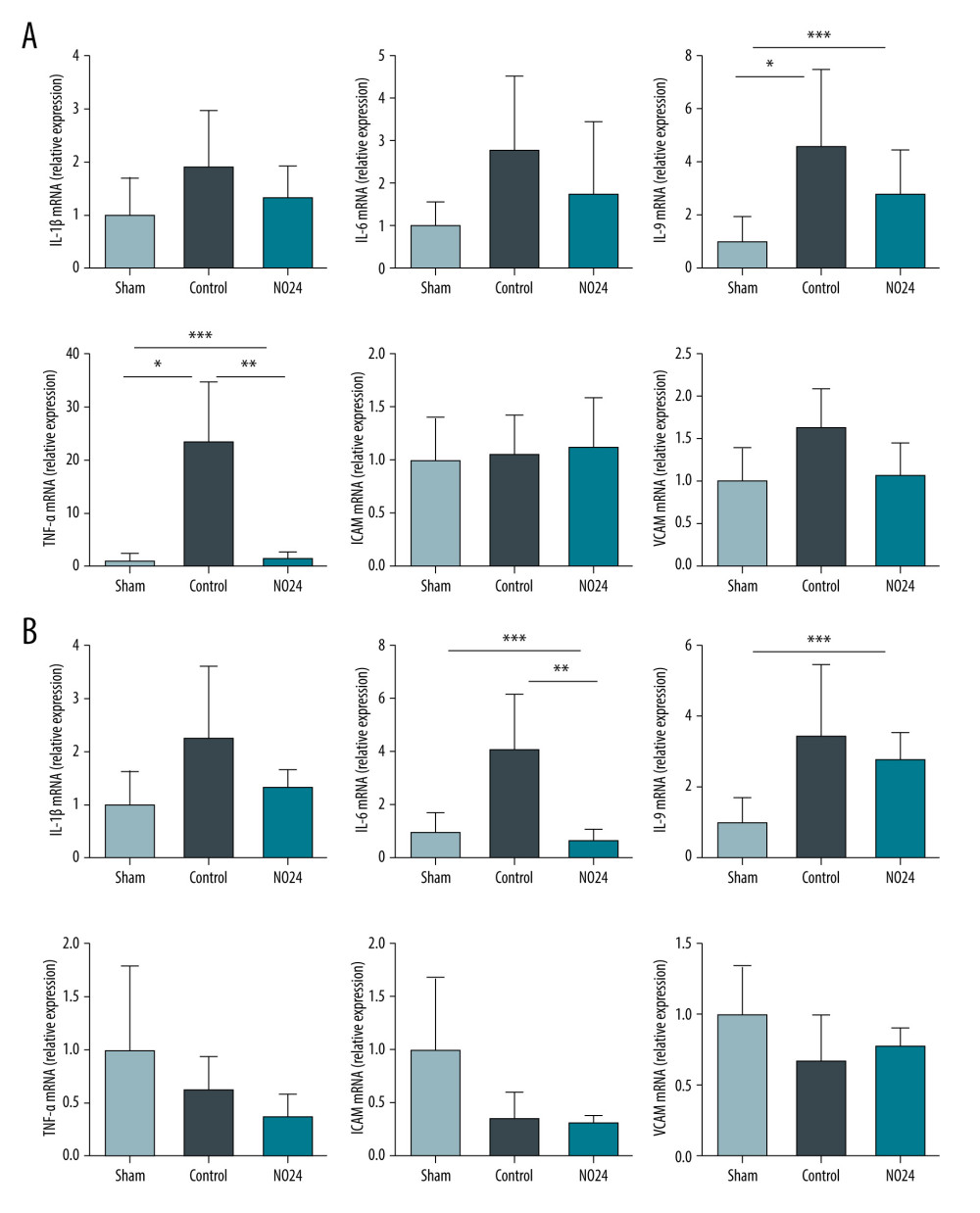 Figure 3. mRNA expression of inflammatory cytokines and adhesion molecules in renal cortex and medullamRNA expression levels were measured by real-time PCR. mRNA expression of inflammatory cytokines (IL-1β, IL-6, IL-9, and TNF-α) and adhesion molecules (ICAM and VCAM). mRNA expression levels of inflammatory cytokines were decreased in the NO24 group compared with those in the control group. mRNA expression of adhesion molecules did not differ among groups in the (A) cortex and (B) medulla. * P<0.05, sham group vs control group, ** p<0.05, control group vs NO24 group, and *** P<0.05, sham group vs control group vs NO24 group were considered as significant. Prism, version 5 (GraphPad Software, Inc, San Diego, CA, USA) was used to create the figure.
Figure 3. mRNA expression of inflammatory cytokines and adhesion molecules in renal cortex and medullamRNA expression levels were measured by real-time PCR. mRNA expression of inflammatory cytokines (IL-1β, IL-6, IL-9, and TNF-α) and adhesion molecules (ICAM and VCAM). mRNA expression levels of inflammatory cytokines were decreased in the NO24 group compared with those in the control group. mRNA expression of adhesion molecules did not differ among groups in the (A) cortex and (B) medulla. * P<0.05, sham group vs control group, ** p<0.05, control group vs NO24 group, and *** P<0.05, sham group vs control group vs NO24 group were considered as significant. Prism, version 5 (GraphPad Software, Inc, San Diego, CA, USA) was used to create the figure. 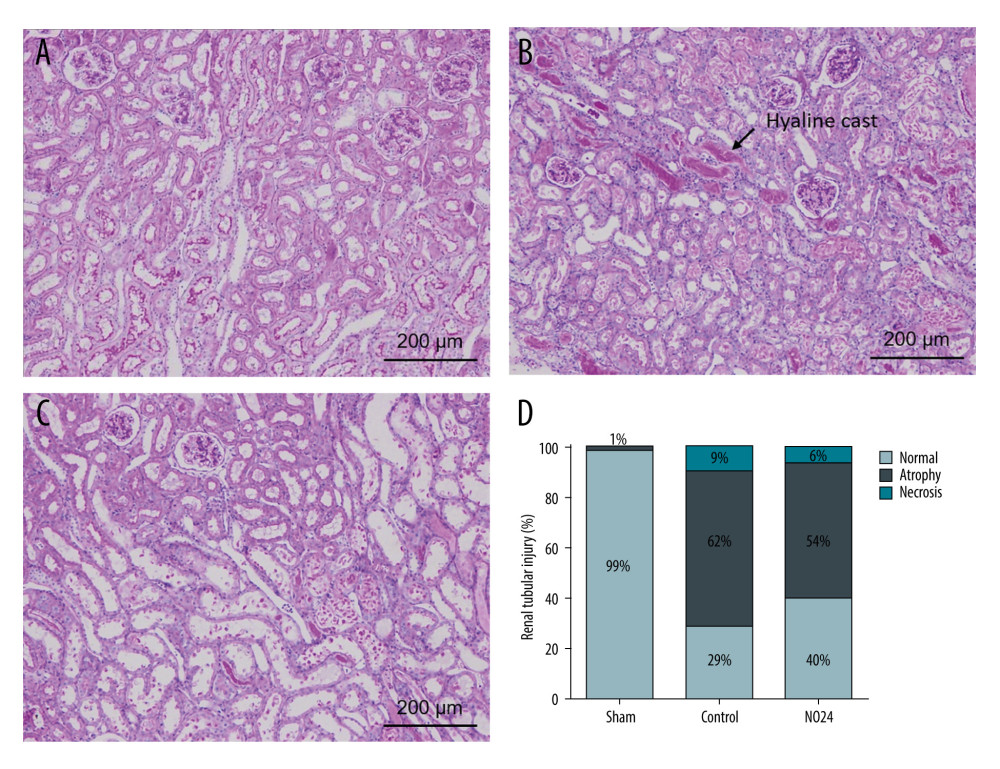 Figure 4. Immunohistochemical analysis of renal morphologyParaffin tissue blocks were prepared for each group by sectioning for immunohistochemical staining. Staining with hematoxylin and eosin and periodic acid-Schiff for renal tissues of (A) sham, (B) control, and (C) NO24 groups (D) and semi-quantitative scoring of tubular damage. Atrophy and necrosis decreased in the NO24 group compared with in the control group. (B) The control group contained many hyaline casts.
Figure 4. Immunohistochemical analysis of renal morphologyParaffin tissue blocks were prepared for each group by sectioning for immunohistochemical staining. Staining with hematoxylin and eosin and periodic acid-Schiff for renal tissues of (A) sham, (B) control, and (C) NO24 groups (D) and semi-quantitative scoring of tubular damage. Atrophy and necrosis decreased in the NO24 group compared with in the control group. (B) The control group contained many hyaline casts. References
1. Ponticelli C, Ischaemia-reperfusion injury: A major protagonist in kidney transplantation: Nephrol Dial Transplant, 2014; 29; 1134-40
2. Grinyo JM, Role of ischemia-reperfusion injury in the development of chronic renal allograft damage: Transplant Proc, 2001; 33; 3741-42
3. Norwood MGA, Bown MJ, Sayers RD, Ischaemia-reperfusion injury and regional inflammatory responses in abdominal aortic aneurysm repair: Eur J Vasc Endovasc Surg, 2004; 28; 234-45
4. Carden DL, Granger DN, Pathophysiology of ischaemia – reperfusion injury: J Pathol, 2000; 190; 255-66
5. Singbartl K, Green SA, Ley K, Blocking P-selectin protects from ischemia/reperfusion-induced acute renal failure: FASEB J, 2000; 14; 48-54
6. Takada M, Nadeau KC, Shaw GD, The cytokine-adhesion molecule cascade in ischemia/reperfusion injury of the rat kidney. Inhibition by a soluble P-selectin ligand: J Clin Invest, 1997; 99; 2682-90
7. Nour M, Scalzo F, Liebeskind DS, Ischemia-reperfusion injury in stroke: Interv Neurol, 2013; 1; 185-99
8. Siriussawakul A, Chen LI, Lang JD, Medical gases: A novel strategy for attenuating ischemia – reperfusion injury in organ transplantation?: J Transplant, 2012; 2012; 819382
9. Lentsch AB, Kato A, Yoshidome H, Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury: Hepatology, 2000; 32; 169-73
10. Shirasugi N, Wakabayashi G, Shimazu M, Up-regulation of oxygen-derived free radicals by interleukin-1 in hepatic ischemia/reperfusion injury: Transplantation, 1997; 64; 1398-1403
11. Ketteler M, Border WA, Noble NA, Cytokines and L-arginine in renal injury and repair: Am J Physiol, 1994; 267; F197-207
12. Phillips L, Toledo AH, Lopez-Neblina F, Nitric oxide mechanism of protection in ischemia and reperfusion injury: J Invest Surg, 2009; 22; 46-55
13. Tarr J, Eggleton P, Winyard P, Nitric oxide and the regulation of apoptosis in tumour cells: Curr Pharm Des, 2006; 12; 4445-68
14. Rodriguez-Pena A, Garcia-Criado FJ, Eleno N, Intrarenal administration of molsidomine, a molecule releasing nitric oxide, reduces renal ischemia-reperfusion injury in rats: Am J Transplant, 2004; 4; 1605-13
15. Barakat N, Hussein AA, Abdel-Maboud M, Ischaemia-reperfusion injury in renal transplantation: The role of nitric oxide in an experimental rat model: BJU Int, 2010; 106; 1230-36
16. Zhu SH, Zhou LJ, Jiang H, Protective effect of indomethacin in renal ischemia-reperfusion injury in mice: J Zhejiang Univ Sci B, 2014; 15; 735-42
17. Bohl Masters KS, Lipke EA, Rice EEH, Nitric oxide-generating hydrogels inhibit neointima formation: J Biomater Sci Polym Ed, 2005; 16; 659-72
18. Kaul S, Cercek B, Rengstrom J, Polymeric-based perivascular delivery of a nitric oxide donor inhibits intimal thickening after balloon denudation arterial injury: Role of nuclear factor-kappaB: J Am Coll Cardiol, 2000; 35; 493-501
19. Huang SL, Kee PH, Kim H, Nitric oxide-loaded echogenic liposomes for nitric oxide delivery and inhibition of intimal hyperplasia: J Am Coll Cardiol, 2009; 54; 652-59
20. Ko HM, Joo SH, Jo JH, Liver-wrapping, nitric oxide-releasing nanofiber downregulates Cleaved Caspase-3 and Bax expression on rat hepatic ischemia-reperfusioniInjury: Transplant Proc, 2017; 49; 1170-74
21. Lee YJ, Kim YR, Jeong WY, Potential protective effect of nitric oxide-releasing nanofibers in hypoxia/reoxygenation-induced cardiomyocyte injury: J Nanosci Nanotechnol, 2019; 19; 6539-45
22. Carreras MC, Poderoso JJ, Mitochondrial nitric oxide in the signaling of cell integrated responses: Am J Physiol Cell Physiol, 2007; 292; C1569-80
23. Salakou S, Kardamakis D, Tsamandas AC, Increased Bax/Bcl-2 ratio up-regulates caspase-3 and increases apoptosis in the thymus of patients with myasthenia gravis: In Vivo, 2007; 21(1); 123-32
24. Thornberry NA, Lazebnik Y, Caspases: Enemies within: Science, 1998; 281; 1312-16
25. Suzuki S, Toledo-Pereyra LH, Interleukin 1 and tumor necrosis factor production as the initial stimulants of liver ischemia and reperfusion injury: J Surg Res, 1994; 57; 253-58
26. Frangogiannis NG, Chemokines in ischemia and reperfusion: Thromb Haemost, 2007; 97; 738-47
27. Franco-Gou R, Roselló-Catafau J, Casillas-Ramirez A, How ischaemic preconditioning protects small liver grafts: J Pathol, 2006; 208; 62-73
28. Martinez-Mier G, Toledo-Pereyra LH, Ward PA, Adhesion molecules in liver ischemia and reperfusion: J Surg Res, 2000; 94; 185-94
29. Martinez-Mier G, Toledo-Pereyra LH, L-selectin and chemokine response after liver ischemia and reperfusion: J Surg Res, 2000; 93; 156-62
30. Waldow T, Witt W, Weber E, Matschke K, Nitric oxide donor-induced persistent inhibition of cell adhesion protein expression and NFκB activation in endothelial cells: Nitric Oxide, 2006; 15; 103-13
Figures
 Figure 1. Serum levels of creatinineSerum creatinine levels were analyzed using a biochemical analyzer at 48 h after surgery. Serum creatinine level increased at 48 h after surgery in all animals that had undergone ischemia-reperfusion. However, it was significantly lower in the NO24 group than in the control group. * P<0.05, sham group vs control group; ** P<0.05, control group vs NO24 group; and *** P<0.05, sham group vs control group vs NO24 group were considered as significant. Prism, version 5 (GraphPad Software, Inc, San Diego, CA, USA) was used to create the figure.
Figure 1. Serum levels of creatinineSerum creatinine levels were analyzed using a biochemical analyzer at 48 h after surgery. Serum creatinine level increased at 48 h after surgery in all animals that had undergone ischemia-reperfusion. However, it was significantly lower in the NO24 group than in the control group. * P<0.05, sham group vs control group; ** P<0.05, control group vs NO24 group; and *** P<0.05, sham group vs control group vs NO24 group were considered as significant. Prism, version 5 (GraphPad Software, Inc, San Diego, CA, USA) was used to create the figure. Figure 2. Expression of apoptosis- and nuclear-related protein in renal cortex and medullamRNA expression was determined by immunoblot analysis. (A) Bax, Bcl-2, cleaved caspase-3, and nuclear protein NF-κB activities in the renal cortex were determined but (B) showed no difference in the medulla. * P<0.05, sham group vs control group; ** P<0.05, control group vs NO24 group; and *** P<0.05, sham group vs control group vs NO24 group were considered as significant. Prism, version 5 (GraphPad Software, Inc, San Diego, CA USA) was used to create the figure.
Figure 2. Expression of apoptosis- and nuclear-related protein in renal cortex and medullamRNA expression was determined by immunoblot analysis. (A) Bax, Bcl-2, cleaved caspase-3, and nuclear protein NF-κB activities in the renal cortex were determined but (B) showed no difference in the medulla. * P<0.05, sham group vs control group; ** P<0.05, control group vs NO24 group; and *** P<0.05, sham group vs control group vs NO24 group were considered as significant. Prism, version 5 (GraphPad Software, Inc, San Diego, CA USA) was used to create the figure. Figure 3. mRNA expression of inflammatory cytokines and adhesion molecules in renal cortex and medullamRNA expression levels were measured by real-time PCR. mRNA expression of inflammatory cytokines (IL-1β, IL-6, IL-9, and TNF-α) and adhesion molecules (ICAM and VCAM). mRNA expression levels of inflammatory cytokines were decreased in the NO24 group compared with those in the control group. mRNA expression of adhesion molecules did not differ among groups in the (A) cortex and (B) medulla. * P<0.05, sham group vs control group, ** p<0.05, control group vs NO24 group, and *** P<0.05, sham group vs control group vs NO24 group were considered as significant. Prism, version 5 (GraphPad Software, Inc, San Diego, CA, USA) was used to create the figure.
Figure 3. mRNA expression of inflammatory cytokines and adhesion molecules in renal cortex and medullamRNA expression levels were measured by real-time PCR. mRNA expression of inflammatory cytokines (IL-1β, IL-6, IL-9, and TNF-α) and adhesion molecules (ICAM and VCAM). mRNA expression levels of inflammatory cytokines were decreased in the NO24 group compared with those in the control group. mRNA expression of adhesion molecules did not differ among groups in the (A) cortex and (B) medulla. * P<0.05, sham group vs control group, ** p<0.05, control group vs NO24 group, and *** P<0.05, sham group vs control group vs NO24 group were considered as significant. Prism, version 5 (GraphPad Software, Inc, San Diego, CA, USA) was used to create the figure. Figure 4. Immunohistochemical analysis of renal morphologyParaffin tissue blocks were prepared for each group by sectioning for immunohistochemical staining. Staining with hematoxylin and eosin and periodic acid-Schiff for renal tissues of (A) sham, (B) control, and (C) NO24 groups (D) and semi-quantitative scoring of tubular damage. Atrophy and necrosis decreased in the NO24 group compared with in the control group. (B) The control group contained many hyaline casts.
Figure 4. Immunohistochemical analysis of renal morphologyParaffin tissue blocks were prepared for each group by sectioning for immunohistochemical staining. Staining with hematoxylin and eosin and periodic acid-Schiff for renal tissues of (A) sham, (B) control, and (C) NO24 groups (D) and semi-quantitative scoring of tubular damage. Atrophy and necrosis decreased in the NO24 group compared with in the control group. (B) The control group contained many hyaline casts. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








