12 November 2021: Original Paper
Coronavirus Disease 2019 (COVID-19) in Solid Organ Transplant Recipients: A Case-Control Study
Alejandro Muñoz Serrano1ABDEF*, Ana Arias2ABDE, Víctor Moreno-TorresDOI: 10.12659/AOT.933152
Ann Transplant 2021; 26:e933152
Abstract
BACKGROUND: It is unclear whether solid organ transplant (SOT) patients have more severe coronavirus disease 2019 (COVID-19) and worse outcome than the general population.
MATERIAL AND METHODS: We conducted a case-control study on 32 SOT recipients and 84 non-SOT controls matched for age and sex admitted for confirmed COVID-19. The primary endpoint was in-hospital all-cause mortality rate. Secondary endpoints included severe acute respiratory distress syndrome (ARDS), use of high-flow oxygen therapy, and length of hospital stay.
RESULTS: The median (IQR) Charlson comorbidity index (CCI) at admission was significantly higher in SOT recipients (6 (3-8) vs 3 (2-4); P<0.01). Fever was less frequent in SOT recipients (78% vs 94%, P=0.01). SOT recipients had a higher median SaO2/FiO2 at admission (452 [443-462] vs 443 [419-452], P<0.01) and reached the worst SaO2/FiO2 value later during hospitalization 15 (10-21) vs 11 (9-14) days, P=0.01). Both groups had a similar severe ARDS rate during hospitalization (33% vs 28%) (p=0.59). There were no significant differences during hospitalization in terms of highest level of respiratory support needed, or length of hospital stay: 8.5 (5.5-21) vs 11.5 (6.5-16.5) days; P=0.34) in SOT recipients when compared to controls. In-hospital all-cause mortality rates were significantly higher in SOT recipients (21.9% vs 4.7%, P<0.01; OR 1.08; 95% CI 0.10-10.98), but among patients who died, median CCI was similar between groups (8 [6-8] vs 7 [6-8]).
CONCLUSIONS: In our experience, hospitalized SOT recipients for COVID-19 had higher in-hospital mortality compared to non-SOT patients, probably due to the greater number of underlying comorbidities, and not directly related to chronic immunosuppression.
Keywords: Liver Transplantation, Heart Transplantation, Kidney Transplantation, COVID-19, Lung Transplantation, severe acute respiratory syndrome coronavirus 2, COVID-19, Case-Control Studies, Humans, Organ Transplantation, SARS-CoV-2, transplant recipients
Background
Coronavirus disease 2019 (COVID-19) was first reported in December 2019 when a new strain of coronavirus was isolated and named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1,2]. Due to the alarming levels of spread and severity of the outbreak, the World Health Organization characterized COVID-19 as a pandemic on March 11th, 2020 [3]. To date, it is reported that 202 608 000 people have been infected with SARS-CoV-2 worldwide and 4 290 000 people have died due to COVID-19 [4]. Studies on the general population suggest that factors linked to severe disease include advanced age, male sex, and underlying comorbidities such as hypertension, obesity, diabetes, chronic kidney disease, chronic lung disease, and coronary heart disease [1,5–9].
Solid organ transplant (SOT) recipients are also at risk of SARS-CoV-2 infection. Whether SOT recipients are at particularly high risk for severe COVID-19 and worse outcome compared with non-transplant (non-SOT) patients is unclear, as the impact of posttransplant chronic immunosuppression on the natural history of COVID-19 is uncertain. On one hand, chronic immunosuppression can increase the viral load, leading to more severe disease, but on the other hand, these drugs can attenuate the inflammatory response linked to cytokine release syndrome [10,11]. Many of the comorbid conditions linked to severe COVID-19 frequently occur among SOT recipients and it is unclear whether these or other potential confounding features, rather than chronic immunosuppression itself, contribute to the risk.
We report the clinical characteristics and outcomes of a cohort of SOT recipients admitted to hospital with COVID-19 compared with a concomitant cohort of non-SOT COVID-19 patients.
Material and Methods
DESIGN OF THE STUDY:
This was a single-center retrospective study of consecutive SOT recipients admitted to hospital for confirmed COVID-19 from March 1 to May 31, 2020. Non-SOT patients admitted to hospital due to COVID-19 during the same study period, matched according to sex and age, were used as controls. According to the World Health Organization (WHO) COVID severity scale, patients to be included in the study must have ≥3 points in the scale [12].
SARS-CoV-2 infection was confirmed by real-time reverse transcription polymerase chain reaction assay (rt-PCR) in nasopharyngeal swabs specimens. The study was approved by the hospital review board (PI134-20) and informed consent was obtained from all patients to include their clinical information within a database for epidemiological and clinical studies.
DATA COLLECTION:
Data were retrospectively collected from electronic medical records, and included demographic variables, past medical history, comorbidities, clinical symptoms, physical examination findings, laboratory and diagnostic imaging tests at admission, treatments, in-hospital complications, length of hospital stay, and outcomes (hospital discharge or death).
Age was divided into 3 groups: ≤60 years, 61–70 years, and ≥71 years. Maximum body temperature was stratified as follows: ≤37.3°C, 37.4–38°C, 38.1–39°C, and ≥39.1°C. Highest level of respiratory support was categorized attending to the maximum request during admission: room air, oxygen supplementation, use of high-flow oxygen therapy or BiPAP, need for mechanical ventilation, and extracorporeal membrane oxygenation (ECMO). In SOT recipients, time from transplant to COVID-19 diagnosis was expressed in years and categorized into 3 groups: <1 year, 1–5 years, and >5 years.
Arterial oxygen saturation and inspiratory oxygen fraction ratio (SaO2/FiO2) was calculated by pulse oximetry. SaO2/FiO2 has a good correlation with the partial pressure of arterial oxygen ratio (PaO2/FiO2) [13]. The worst registry of respiratory situation on medical records during hospitalization was defined by the highest level of respiratory support that the patient needed. Time from symptom onset to the highest respiratory support date was measured in days.
OUTCOMES AND STUDY DEFINITIONS:
The primary endpoint was in-hospital all-cause mortality. Secondary endpoints included severe acute respiratory distress syndrome (ARDS) defined as SaO2/FiO2 ratio of 148 (PaO2/FiO2 ratio of 100) [14,15], and length of hospitalization. The outcomes from SOT recipients were compared with those of non-SOT patients.
IMMUNOSUPPRESSIVE TREATMENT APPROACH:
According to the general approach in our center, blood levels of tacrolimus, cyclosporine, everolimus, and sirolimus levels were reduced or mantained, and mycophenolate mofetil/mycophenolic acid was reduced or temporally discontinued according to the criteria of the treating physician. The baseline dose of steroids was not modified.
STATISTICAL ANALYSIS:
Quantitative variables are expressed as medians with interquartile ranges (IQR) and qualitative variables as counts and percentages. The Mann-Whitney U test and chi-square test were used to compare differences between the SOT recipient group and the non-SOT recipient group, as appropriate. The association of transplantation and the primary endpoint was assessed through conditional logistic regression to compute odds ratios (ORs) and their 95% confidence intervals (CI). The statistical significance level was set at two-sided
Results
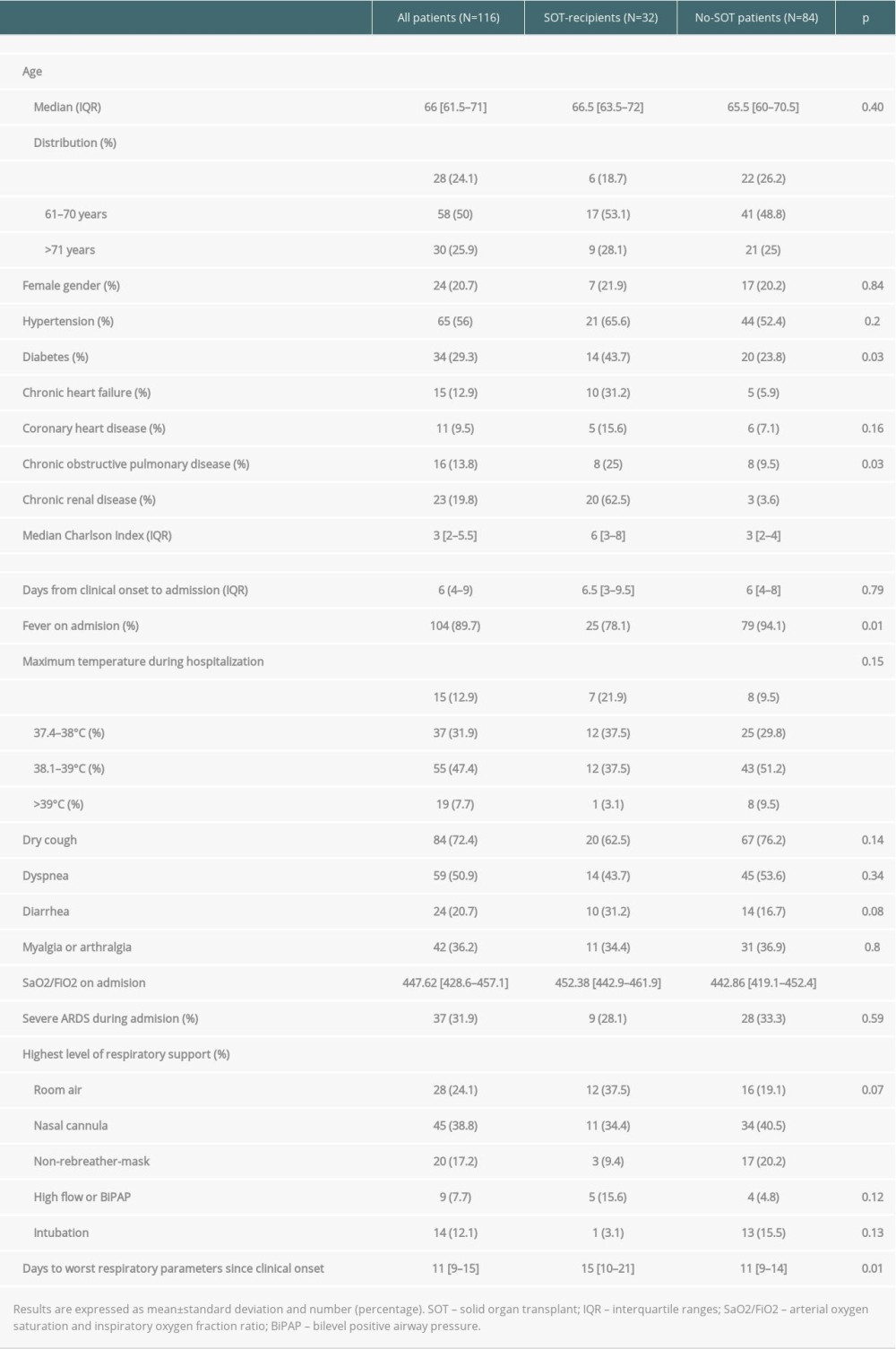
We identified 1494 consecutive adult patients hospitalized with confirmed COVID-19 in our center during the study period. Thirty-two (2.1%) of the 1494 patients were SOT recipients (78.1% males, median age 66.5 years [IQR 63.5–72]). Of the remaining 1462 patients, 84 non-SOT patients that were not under immunosuppressive therapy, matched according to sex and age, were used as controls (79.8% males, median age 65.5 years [IQR 60.5–70.5],
The median time from symptoms onset to hospital admission was 6 days (IQR 4–9), without differences between groups. The median Charlson comorbidity index (CCI) for the overall study population was 3 (IQR 2–5.5). Median CCI was significantly higher in the SOT recipients (6 [IQR 3–8] vs 3 [IQR 2–4];
The most common presenting symptoms were fever, cough, and dyspnea. Fever was less frequent in SOT recipients (78% vs 94%,
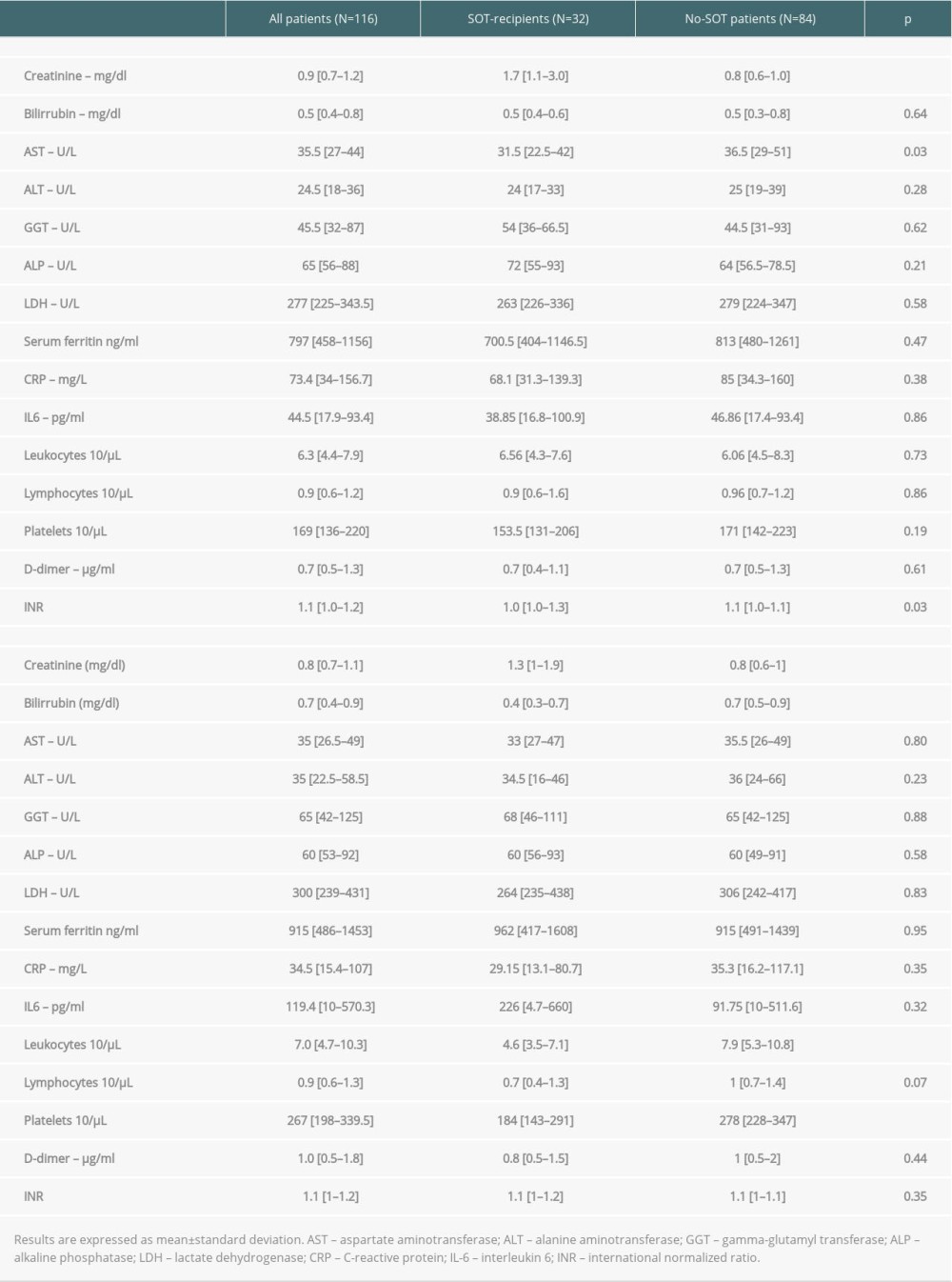
Biochemical findings upon admission are shown in Table 2. Significant differences between SOT recipients and controls were found for serum creatinine (1.7 mg/dl [1.1–3.0 mg/dl]) vs 0.8mg/dl [0.6–1.0 mg/dl],
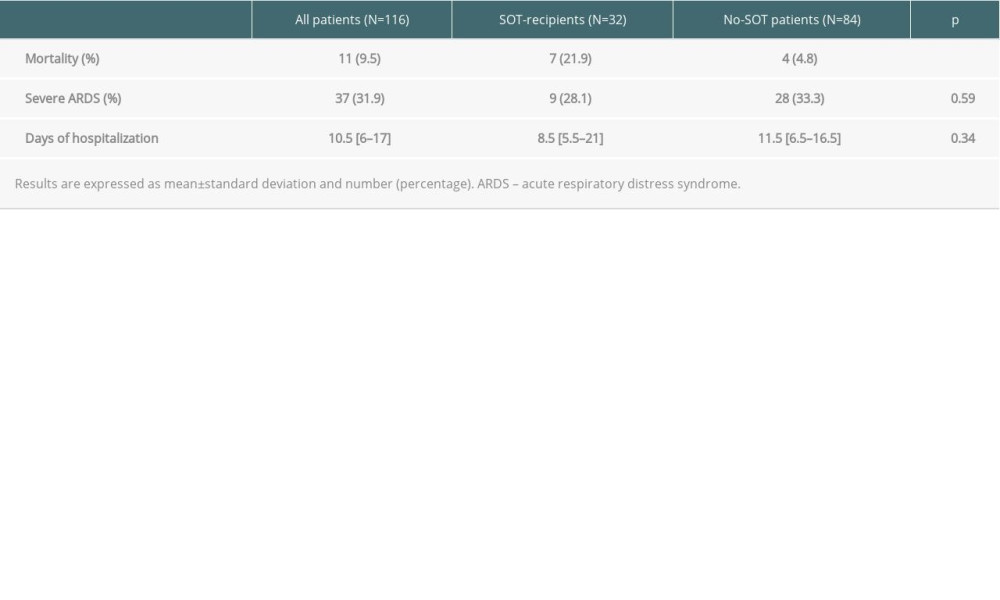
Severe ARDS was developed in 37 (31.9%) patients overall and was similar between the 2 groups (SOT recipients 28.1% vs 33.3%,
The in-hospital all-cause mortality rate was significantly higher in SOT recipients than in the control group (21.9% vs 4.7%,
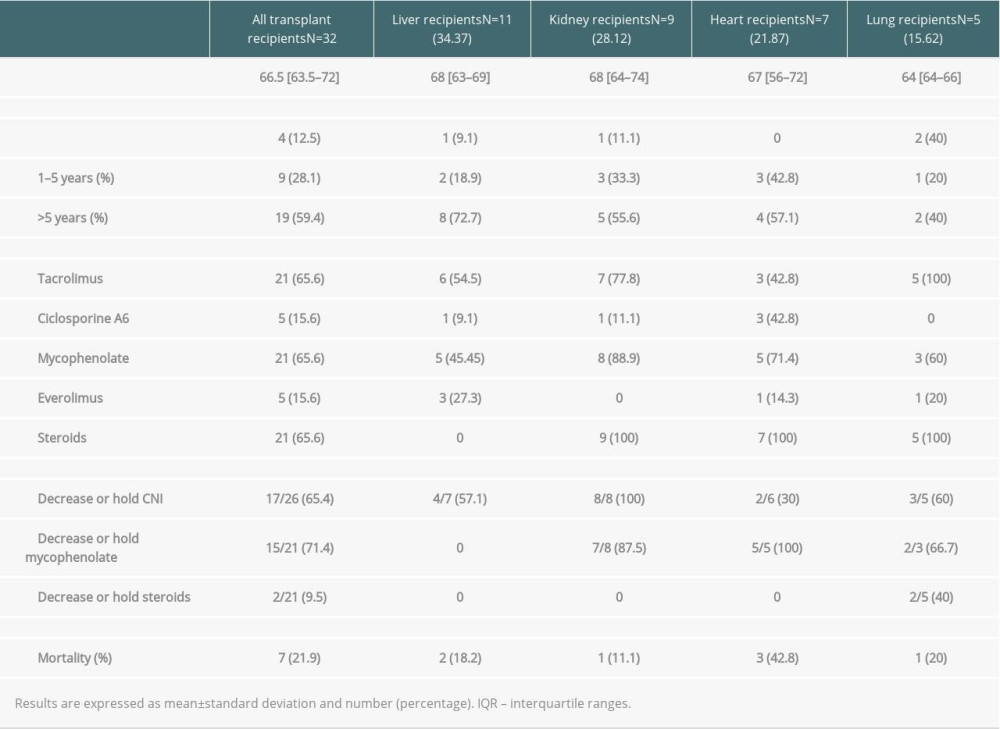
Among SOT recipients, 11 (34%) received a liver, 9 (28%) received a kidney, 7 (22%) received a heart, and 5 (16%) received a lung. The median time from transplant to hospital admission for COVID-19 was shorter in lung transplant recipients (3.13 years [IQR 0.68–9.85]) and longer in liver transplant recipients (13.25 [IQR 3.71–15.94] years). Baseline immunosuppression regimen and management of immunosuppressive drugs after COVID-19 diagnosis are described in Table 4. Tacrolimus (n=21; 65.6%), mycophenolate mofetil (n=21; 65.6%), and steroids (n=21; 65.6%) were the predominant immunosuppressants. Among COVID-19 diagnosis, immunosuppressive regimens were modified in all patients. Tacrolimus dose was reduced in 10 of the 21 patients, discontinued in 4 patients, and remained unchanged in 7 patients. Cyclosporine A dose was reduced in 1 out of 5 patients, discontinued in 1 patient, and unchanged in 3 patients. Mycophenolate mofetil dose was reduced in 6 patients and discontinued in 15 of the 21 patients (71%). There were no graft rejection episodes during the study period.
Discussion
In the present study, days elapsed from symptoms onset to hospital admission were comparable between groups, which allowed us to compare COVID-19 disease evolution over time in SOT recipients and in controls. SOT recipients were less likely to have fever at admission (78% vs 94%) and had lower body temperature values during hospitalization. This fact is well known in SOT recipients, and it is related to the anti-inflammatory effect of immunosuppressive drugs. Dyspnea, cough, inflammatory biochemical parameters values at admission, and risk of developing severe ARDS in SOT recipients were similar to controls. Elapsed time from admission to worst SaO2/FiO2 value during hospitalization was greater in SOT recipients (15 days [IQR10–21] vs 11 days [IQR 9–14];
The in-hospital mortality rate in SOT recipients in our series was 21.9%, similar to the rates of 4.8–37% described by others [16–36]. To date, several articles related with COVID-19 mortality in transplant patients have been published, but their results are variable and heterogeneous. Discarding the studies that analyzed a single organ type of transplant and hematopoietic transplant, most of them demonstrate high mortality rates [17–24]. However, it is noteworthy that some of these studies did not compare their results with the non-transplanted population [17–20], while others did not find such high mortality rates or any differences compared with the non-transplanted population [16,25–30]. Mortality rates in these series were affected by age, number of underlying comorbidities, and by the population analyzed (hospitalized patients only [16,21,24,30] or also outpatients [17–20,22–23,25–28]). In our opinion, inclusion of outpatients can bias the comparison between both groups, since transplant patients are usually closely monitored, which makes it more likely to diagnose mild or asymptomatic cases. The study and follow-up periods were also heterogeneous among publications, which makes interpretation of results difficult, especially considering how fast the protocols were changing for this group of patients in recent months. Some studies adjusted the comparison of groups according to their comorbidities but not to the Charlson index [23]. In our series, mortality during admission was almost 4 times higher in SOT recipients (21.9% vs 4.7%). The lower mortality rate observed in our study in non-SOT patients compared to other series could be explained by the low number of patients between 71 and 79 years (25%) and older than 80 years (4.16%) in our cohort. Mortality for these age groups is closer to that reflected in other series, at 12% and 40%, respectively [31].
To date, some studies suggested that elapsed time from transplantation is a risk factor linked to higher mortality in patients hospitalized for COVID-19 [32–34], with higher mortality in patients transplanted more than 10 years ago, with reduced immunosuppression, higher comorbidities, and age over 65 years [34]. In our series, of the 7 dead SOT patients, 29% had been transplanted less than 5 years ago, 14% 5–10 years ago, and 57% more than 10 years ago. This higher mortality in long-term survival SOT recipients could be explained by the high rate of comorbidities in this population, conditioned by chronic immunosuppressive treatment and other pathologies, some of which were the direct cause of the need for transplant. Some studies have shown a worse course of infection as the CCI increases [35]. In our series, among the 7 SOT recipients who died, the median CCI was 8 points (6–8) and 4 were older than 70 years. Non-SOT patients who died had a median of 7 points (6–8) in CCI, and 2 out of 4 were older than 70 years. All of them had severe ARDS.
The strengths of this study are that the same criteria were used for all the patients, for hospitalization and similar treatment strategy, and modifications in immunosuppressant treatment. Nevertheless, we are aware that our study suffers from some limitations, mainly due to the retrospective design and the small number of SOT recipients, which prevent forming strong conclusions on the efficacy of the optimal management of immunosuppression in SOT recipients with COVID-19.
Conclusions
Despite the published literature, there are still many gaps in knowledge about the relationship between COVID-19 and solid organ transplantation. There is no doubt that these are high-risk patients due to their close contact with the hospital environment, but the role of immunosuppression and the best management options are still unknown. The results in different publications regarding mortality due to COVID-19 in transplant patients are heterogeneous, probably due to the difficulty of knowing the degree of immunosuppression of each patient and the different treatment regimens available. We contribute with a new series of admitted transplant patients compared with non-transplanted patients of the same age and sex during a period of time in which the admission criteria and the management protocol were the same for both groups.
In our experience, SOT recipients hospitalized for COVID-19 had higher in-hospital mortality compared to non-SOT patients, probably due to greater underlying comorbidities and not directly related to chronic immunosuppression. No differences were found between groups in clinical course, severe ARDS, length of hospital stay, and highest level or respiratory support needed. However, the patients who died in both groups had an elevated and similar CCI, suggesting the high mortality risk of comorbidity. In our series, the CCI was higher in transplant patients, which could explain the higher mortality in this group.
References
1. Zhu N, Zhang D, Wang W, A novel coronavirus from patients with pneumonia in China, 2019: N Engl J Med, 2020; 382(8); 727-33
2. Wu F, Zhao S, Yu B, A new coronavirus associated with human respiratory disease in China: Nature, 2020; 579(7798); 265-69
3. WHO: WHO Bulletin, 2020; 2019 Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
4. : Weekly Epidemiological and Operational updates December 2020 Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
5. Zhou F, Yu T, Du R, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study: Lancet, 2020; 395(10229); 1054-62
6. Wang D, Hu B, Hu C, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China: JAMA, 2020; 323; 1061-69
7. Li Q, Guan X, Wu P, Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia: N Engl J Med, 2020; 382(13); 1199-207
8. Huang C, Wang Y, Li X, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China: Lancet, 2020; 395(10223); 497-506
9. Guan W, Ni Z, Hu Y, Clinical characteristics of coronavirus disease 2019 in China: N Engl J Med, 2020; 382(18); 1708-20
10. Ritchie AI, Singanayagam A, Immunosuppression for hyperinflammation in COVID-19: A double-edged sword?: Lancet, 2020; 395; 1111
11. Mehta P, McAuley DF, Brown M, COVID-19: Consider cytokine storm syndromes and immunosuppression: Lancet, 2020; 395(10229); 1033-34
12. World Health Organization
13. Rice TW, Wheeler AP, Bernard GRNational Institutes of Health, National Heart, Lung, and Blood Institute ARDS Network, Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS: Chest, 2007; 132(2); 410-17
14. Ranieri VM, Rubenfeld GD, Thompson BTARDS Definition Task Force, Acute respiratory distress syndrome: The Berlin Definition: JAMA, 2012; 307; 2526-33
15. Wu C, Chen X, Cai Y, Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China: JAMA Intern Med, 2020; 180(7); 934-43
16. Avery RK, Chiang TPY, Marr KA, Inpatient COVID-19 outcomes in solid organ transplant recipients compared to non-solid organ transplant patients: A retrospective cohort: Am J Transplant, 2021; 21(7); 2498-508
17. Pereira MR, Mohan S, Cohen DJ, COVID-19 in solid organ transplant recipients: Initial report from the US epicenter: Am J Transplant, 2020; 20(7); 1800-8
18. Fernández-Ruiz M, Andrés A, Loinaz C, COVID-19 in solid organ transplant recipients: A single-center case series from Spain: Am J Transplant, 2020; 20(7); 1849-58
19. Yi SG, Rogers AW, Saharia A, Early experience with COVID-19 and solid organ transplantation at a US high-volume transplant center: Transplantation, 2020; 104(11); 2208-14
20. Søfteland JM, Friman G, von Zur-Mühlen B, COVID-19 in solid organ transplant recipients: A national cohort study from Sweden: Am J Transplant, 2021; 21(8); 2762-73
21. Miarons M, Larrosa-García M, García-García S, COVID-19 in solid organ transplantation: a matched retrospective cohort study and evaluation of immunosuppression management: Transplantation, 2021; 105(1); 138-50
22. Trapani S, Masiero L, Puoti F, Incidence and outcome of SARS-CoV-2 infection on solid organ transplantation recipients: A nationwide population-based study: Am J Transplant, 2021; 21(7); 2509-21
23. Fisher AM, Schlauch D, Mulloy M, Outcomes of COVID-19 in hospitalized solid organ transplant recipients compared to a matched cohort of non-transplant patients at a national healthcare system in the United States: Clin Transplant, 2021; 35(4); 1-12
24. Nair V, Jandovitz N, Hirsch JS, An early experience on the effect of solid organ transplant status on hospitalized COVID-19 patients: Am J Transplant, 2021; 21(7); 2522-31
25. Tschopp J, L’Huillier AG, Mombelli M, First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study: Am J Transplant, 2020; 20(10); 2876-82
26. Hadi YB, Naqvi SFZ, Kupec JT, Outcomes of COVID-19 in solid organ transplant recipients: A propensity-matched analysis of a large research network: Transplantation, 2021; 105(6); 1365-71
27. Arya A, Li M, Aburjania N, COVID-19 in solid organ transplantation: Disease severity and clinical update: Transplant Proc, 2021; 53(4); 1227-36
28. Linares L, Cofan F, Diekmann F, A propensity score-matched analysis of mortality in solid organ transplant patients with COVID-19 compared to non-solid organ transplant patients: PLoS One, 2021; 16(3); e0247251
29. Chaudhry ZS, Williams JD, Vahia A, Clinical characteristics and outcomes of COVID-19 in solid organ transplant recipients: A cohort study: Am J Transplant, 2020; 20(11); 3051-60
30. Pereira MR, Arcasoy S, Farr MA, Outcomes of COVID-19 in solid organ transplant recipients: A matched cohort study: Transpl Infect Dis, 2021; 23(4); e13637
31. Casas-Rojo JM, Clinical characteristics of patients hospitalized with COVID-19 in Spain: Results from the SEMI-COVID-19 Registry: Rev Clin Esp (Barc), 2020; 220(8); 480-94
32. Belli LS, Duvoux C, Karam V, COVID-19 in liver transplant recipients: Preliminary data from the ELITA/ELTR registry: Lancet Gastroenterol Hepatol, 2020; 5(8); 724-25
33. Webb GJ, Moon AM, Barnes E, Determining risk factors for mortality in liver transplant patients with COVID-19: Lancet Gastroenterol Hepatol, 2020; 5(7); 643-44
34. Becchetti C, Zambelli MF, Pasulo L, COVID-19 in an international European liver transplant recipient cohort: Gut, 2020; 69(10); 1832-40
35. Tuty Kuswardhani RA, Henrina J, Pranata R, Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: A systematic review and meta-analysis: Diabetes Metab Syndr Clin Res Rev, 2020; 14(6); 2103-9
In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860