16 April 2021: Original Paper
Efficacy and Safety of Once-Daily LCP-Tacrolimus Versus Twice-Daily Immediate-Release Tacrolimus in Adult Hispanic Stable Kidney Transplant Recipients: Sub-Group Analysis from a Phase 3 Trial
Arman Faravardeh1DE*, Sanjeev Akkina2DE, Rafael Villicana3DE, Giselle Guerra4DE, Misbah A. Moten5ADE, Ulf Meier-Kriesche5ADE, Daniel R. Stevens5ACDE, Samir J. Patel5DE, Suphamai Bunnapradist6DEDOI: 10.12659/AOT.929535
Ann Transplant 2021; 26:e929535
Abstract
BACKGROUND: The pharmacokinetics and metabolism of tacrolimus, an immunosuppressant commonly used to prevent transplant rejection, can differ in specific subpopulations. This analysis examined treatment outcomes and safety of immediate-release tacrolimus (IR-Tac) and LCP-tacrolimus (LCPT) in stable Hispanic kidney transplant recipients.
MATERIAL AND METHODS: This was a post hoc analysis of clinical trial data from Hispanic adult stable kidney transplant recipients randomized to remain on IR-Tac or convert from IR-Tac to a reduced dose of LCPT (NCT00817206). Composite treatment failure was evaluated at 12 months. Estimated glomerular filtration rate and tacrolimus trough concentrations were evaluated over 12 months.
RESULTS: Fifty-five stable (LCPT n=26, IR-Tac n=29) kidney transplant recipients who self-identified as Hispanic or Latino were included in this analysis. Composite treatment failure occurred in 1 patient (4%) who converted to LCPT and 1 (3%) who remained on IR-Tac. The estimated glomerular filtration rate was stable over time and similar in the 2 treatment groups (P=0.08). Tacrolimus trough levels for both groups were similar over time in the 2 treatment groups (P=0.98). Treatment-emergent adverse events were similar in patients who converted to LCPT and in those who remained on IR-Tac.
CONCLUSIONS: Efficacy and safety were similar in Hispanic kidney transplant recipients who converted from IR-Tac to LCPT and in those remaining on IR-Tac.
Keywords: Glomerular Filtration Rate, Graft Rejection, Kidney Transplantation, Safety, Tacrolimus, Drug Administration Schedule, Hispanic or Latino, Immunosuppressive Agents, transplant recipients
Background
Tacrolimus, a commonly used immunosuppressive agent for preventing and treating kidney transplant rejection, has a narrow therapeutic window [1,2]. Because of this and substantial intra-patient variability in tacrolimus pharmacokinetics, individualized dosing is needed to ensure efficacy while minimizing toxicity [1–3]. Due to differences in bioavailability, dosing recommendations also differ between tacrolimus formulations, which include twice-daily immediate-release tacrolimus (IR-Tac), a once-daily extended-release capsule formulation, and a once-daily MeltDose® (LCPT) formulation [1]. For example, owing to the approximately 30% greater bioavailability of LCPT than IR-Tac [4,5], the total daily dose (TDD) must be reduced in patients who switch from IR-Tac to LCPT.
The pharmacokinetics and metabolism of tacrolimus can also differ in subpopulations with a CY3A5*1 allele, and, for instance, can occur at a higher frequency in patients with a West African descent [6–8]. Therefore, these patients may require higher doses of tacrolimus than other individuals to achieve similar trough concentrations [6–9]. A phase 3 trial in de novo transplant recipients found that, although the rate of treatment failure did not differ between LCPT and IR-Tac in the overall population, it was lower with LCPT than IR-Tac in Black patients [10]. This difference was also found in a pooled analysis of phase 3 trials [11].
Analyses from phase 3 data were previously conducted in certain subgroups based on sex, age, and race, to assess the safety and efficacy of LCPT versus IR-tac [10,11]; however, the Hispanic population was not included in these analyses. Research among Hispanics is warranted, since this is the largest minority group in the US (18.3% in 2019) [12], and Hispanics are 1.5 times as likely as non-Hispanics to develop end-stage renal disease [13]; further, they account for an increasing proportion of adults on waiting lists for kidney transplants (20% in 2018) [14]. The CYP3A5*3 allele [15], a loss-of-function mutant [16], is known to slow tacrolimus clearance [17, 18], and the allelic frequency of CYP3A5*3 is lower in Hispanic patients than in White patients. The likelihood of a more rapid metabolism of tacrolimus in Hispanics may predispose these patients to difficulty in attaining trough levels, which can complicate treatment.
Literature currently exists on intravenous tacrolimus and oral IR-Tac, which describes pharmacokinetics, dose requirements, and treatment outcomes compared between Hispanic and non-Hispanic kidney transplant recipients [19–22]. However, dosing and outcomes for IR-Tac and LCPT have not yet been reported in Hispanic kidney transplant recipients [19]. Here, we examined the outcomes, kidney function, and safety of IR-Tac and LCPT in Hispanic/Latino patients included in a phase 3 clinical trial of stable kidney transplant recipients.
Material and Methods
STUDY DESIGN:
This was a post hoc analysis of data from patients who self-identified as Hispanic or Latino in a phase 3 clinical trial in stable kidney transplant recipients (NCT00817206). The clinical trial was approved by the local health authority and Ethics Committee or Institutional Review Board and was conducted in accordance with the International Council for Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. Informed consent was obtained from all patients.
Details of the study design and overall findings were previously published [23]. Briefly, the study was a 12-month, 2-armed, parallel group, prospective, randomized, open-label, multicenter phase 3 controlled trial to establish the efficacy and safety of switching treatment from IR-Tac to LCPT in stable adult kidney transplant recipients. The study was conducted at 47 sites in the US between December 23, 2008, and February 7, 2011. Stable adult (≥18 years) kidney transplant recipients who received a transplant from 3 months to 5 years prior to the time of screening were eligible for enrollment if they were on a stable tacrolimus dose with tacrolimus trough levels within 4 to 15 ng/mL. A stable twice-daily tacrolimus dose was defined as that which was unchanged for ≥30 days at the time of screening. Patients were excluded if they had received another organ or bone marrow transplant; had an estimated glomerular filtration rate (eGFR) <30 mL/min at screening; a white blood cell count ≤2.8×109/L (unless the white blood cell count had been stable for at least 2 weeks); had received sirolimus or everolimus 3 months prior to enrollment; or had results that were outside of laboratory reference ranges.
Eligible patients with a stable target trough concentration of 4 to 15 ng/mL for IR-Tac at enrollment were randomized to either remain on IR-Tac or to switch from IR-Tac to LCPT for up to 12 months. For non-Black patients, initial dosing of LCPT was 0.7 times the TDD of tacrolimus being taken before conversion to account for the increased bioavailability of LCPT. For Black patients, initial dosing of LCPT was 0.85 times the TDD of tacrolimus being taken before conversion to account for the higher doses of tacrolimus needed to achieve comparable blood concentrations in Black patients [9]. During the study, tacrolimus trough concentrations were used to adjust the dose to maintain trough levels of 4 to 15 ng/mL.
Tacrolimus trough concentrations were measured by a central laboratory in whole blood samples drawn ≤30 min before morning doses of study medication. Following protein precipitation of whole blood samples and solid-phase chromatography, tacrolimus concentrations were measured by reverse-phase high performance liquid chromatography-tandem mass spectroscopy. An XTerra MS C8, 2.1×100 mm, 5-μm column (Waters, Millford, MA, USA) was used, and the mobile phase was 90: 10 (v/v) methanol: 0.002 M ammonium acetate in 0.1% formic acid. Rapamycin was used as the internal standard. Detection was in positive ion mode (m/z 821.500→768.450 for tacrolimus and m/z 931.570→864.480 for rapamycin). Results were quantified using the peak area ratio method. The TDD of tacrolimus was calculated as the sum in milligrams of LCPT or IR-Tac.
STUDY ENDPOINTS:
The primary endpoint of this analysis was composite treatment failure, and secondary endpoints included individual components of the composite, TDD, renal function, and safety outcomes. Composite treatment failure was defined as an occurrence of one of the following within 12 months: biopsy-proven acute rejection (BPAR), death, graft loss, or loss to follow-up within 12 months after the randomization date. BPAR was assessed by a blinded central pathologist and defined as Banff grade ≥1A, based on Banff 2007 criteria [24]. Renal function was estimated by eGFR using the Modification of Diet in Renal Disease 7 formula [25,26].
Safety assessments included the incidence of treatment-emergent adverse events (TEAEs), treatment-related TEAEs (in the opinion of the investigator), and post-transplant diabetes mellitus in patients without evidence of preexisting diabetes (diabetes at baseline or a medical history of diabetes, baseline fasting plasma glucose ≥126 mg/dL, prior use of a hypoglycemic agent or insulin for diabetes, or HbA1c ≥6.5% before transplantation).
STATISTICAL ANALYSES:
This post hoc analysis was limited to patients in the trial who self-identified as Hispanic or Latino and who received at least 1 dose of study drug. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Categorical variables were compared by chi-square test, and continuous variables by analysis of variance. Composite treatment failure and individual components of it (BPAR, death, graft loss, and loss to follow-up) were compared between LCPT and IR-Tac groups by Fisher’s exact test. Mean tacrolimus daily dose, trough concentrations, and eGFR over time were compared between treatment groups using a general linear model for repeated measures. The general linear model was used to evaluate treatment and the day of evaluation as independent variables, which was used to compute a
Results
PATIENTS:
Of the 326 patients randomized in the stable transplant recipient study, 55 self-identified as Hispanic or Latino (26 were randomized to LCPT and 29 were randomized to IR-Tac), 48 of whom completed the study (Figure 1). Baseline demographics and clinical characteristics for the study are summarized in Table 1. Briefly, most participants in the Hispanic/Latino subpopulations were men, and the average age was 46.2±13.0 years. The average time between transplant to enrollment was 21.0±15.6 months, and 66% received a transplanted kidney from a deceased donor. Baseline characteristics within the Hispanic subpopulation did not differ significantly between patients in the LCPT and IR-Tac groups.
TDD AND TACROLIMUS TROUGH LEVELS:
The mean TDD of tacrolimus at enrollment was similar in patients who switched from IR-Tac to LCPT (5.79±4.24 mg) and in those who remained on IR-Tac (5.45±4.21 mg). In both groups, TDD decreased gradually over time: at month 12, the mean TDD had decreased to 4.26±4.76 mg for patients who switched from IR-Tac to LCPT and to 4.70±3.34 mg for patients who remained on IR-Tac (Figure 2A). The trends in TDD over time did not significantly differ between the LCPT and IR-Tac groups (P=0.94). Tacrolimus trough levels for both groups were also similar over time in the 2 treatment groups (P=0.98) (Figure 2B), and there were no significant trends over the 12-month study period (P=0.84) (Figure 2C).
RENAL FUNCTION:
Renal function, approximated using the eGFR, changed little and was similar over time in the 2 treatment groups (P=0.08) (Figure 3).
EFFICACY OUTCOMES:
Treatment failure occurred in 1 (4%) Hispanic/Latino patient in the LCPT group, due to BPAR, and in 1 (3%) in the IR-Tac group, due to loss to follow-up (Table 2). One LCPT patient experienced a grade 1B acute cellular rejection episode at study day 57 with a tacrolimus concentration of 3.4 ng/mL just prior to BPAR diagnosis; 1 IR-Tac patient was lost to follow-up at 357 days after randomization. A forest plot depicting composite treatment failure and individual efficacy endpoints for LCPT versus IR-Tac among Hispanic and non-Hispanic groups is shown in the Supplementary Figure 1.
SAFETY:
Drug-related TEAEs were reported for 7 Hispanic/Latino patients (27%) in the LCPT group and 4 (14%) in the IR-Tac group. The most common drug-related TEAEs were urinary tract infection (n=1 [4%] LCPT, n=2 [7%] IR-Tac), diarrhea (n=1 [4%] LCPT, n=1 [3%] IR-Tac), and increased serum creatinine (n=1 [4%] LCPT, n=1 [3%] IR-Tac) (Table 3). In patients without a prior history of diabetes, post-transplant diabetes mellitus occurred in 0% (0/13) of patients in the LCPT group and 6% (1/18) of patients in the IR-Tac group. No deaths or malignancies occurred in either treatment group.
According to the study investigators, 3 patients on LCPT discontinued for the following reasons: intubation prevented administration of oral medications (1), mild tacrolimus toxicity (1), and acute rejection (1).
Discussion
Hispanics are at higher risk than non-Hispanics for developing kidney failure [13] and are therefore more likely to require kidney transplantation. The current analysis showed that treatment failure rates, kidney function, and safety were similar in Hispanic kidney transplant recipients that either converted from IR-Tac to LCPT or remained on IR-Tac. This aligns with results from the full population from the same trial [23] and in the full population in a trial in de novo kidney transplant recipients [10,11]. Further, new post-transplant diabetes mellitus, an infrequent but serious adverse effect associated with tacrolimus [27], was not observed in Hispanic transplant recipients who converted to LCPT. These differences in development of new-onset diabetes after transplantation were not significantly different between the 2 groups.
Owing to the greater bioavailability of LCPT than IR-Tac, a reduced TDD is usually observed in patients who convert from IR-Tac to LCPT. A recent retrospective study of stable kidney recipients who switched from IR-Tac to LCPT found 34% greater bioavailability of LCPT [5]. Similarly, a randomized clinical trial in de novo kidney transplant recipients found 30% greater bioavailability for LCPT [4]. In the current study, to account for the greater bioavailability of LCPT, conversion to LCPT occurred at 0.7 to 0.85 times that of the IR-tac dose taken prior to conversion. This resulted in similar tacrolimus trough concentrations in the LCPT and IR-Tac treatment groups throughout the study.
Although the current study did not determine the frequency of CYP3A5*1 and CYP3A5*3 alleles occurring in transplant recipients, other studies have examined tacrolimus dose requirements in Mexican patients with these polymorphisms. CYP3A5*1, the wild-type allele, occurs in 25% of Hispanic patients [28] and these individuals may require a higher dose of tacrolimus [21]. For instance, the median dose of tacrolimus 0.16 mg/kg/day was required for patients with the CYP3A5*1*1 genotype, and the median dose of 0.07 mg/kg/day was needed for patients with CYP3A5*3*3 [21]. Compared to non-Hispanic patients, over half of Mexican renal patients (N=291) had the CYP3A5*3*3 genotype and required a lower tacrolimus dose than did patients with the CYP3A5*1 allele. Further research is needed to confirm these results in Hispanic/Latino kidney transplant recipients to guide dosing practices among clinicians.
This study has some limitations. The study included a small sample of transplant recipients who were stable at 2 years after transplantation, and the small sample limited the ability to detect differences and make inferences; however, the current study suggests that Hispanic kidney transplant recipients can be safely converted from LCPT to IR-Tac. This study did not compare Hispanic/Latino patients with non-Hispanic subpopulations, and patients were included in the analysis based on self-reported Hispanic/Latino ethnicity. However, these limitations should not have affected the conclusions of this study. Of the small numbers of patients, 5 IR-Tac and 3 LCPT patients were missing tacrolimus levels at several of the later timepoints, and the study did not assess the potential effects of genetic (CYP3A5 alleles) factors, although they are known to impact outcomes in patients treated with tacrolimus. Future research may include conducting a study with genotyping among a larger sample of Hispanic transplant recipients to further explain tacrolimus dose requirements.
Conclusions
In conclusion, this post hoc analysis suggested that in stable Hispanic/Latino kidney transplant recipients, switching from LCPT is as effective and safe as remaining on IR-Tac for preventing treatment failure. This information should help guide clinicians on switching their kidney transplant recipients from twice-daily IR-Tac to once-daily LCPT.
Figures
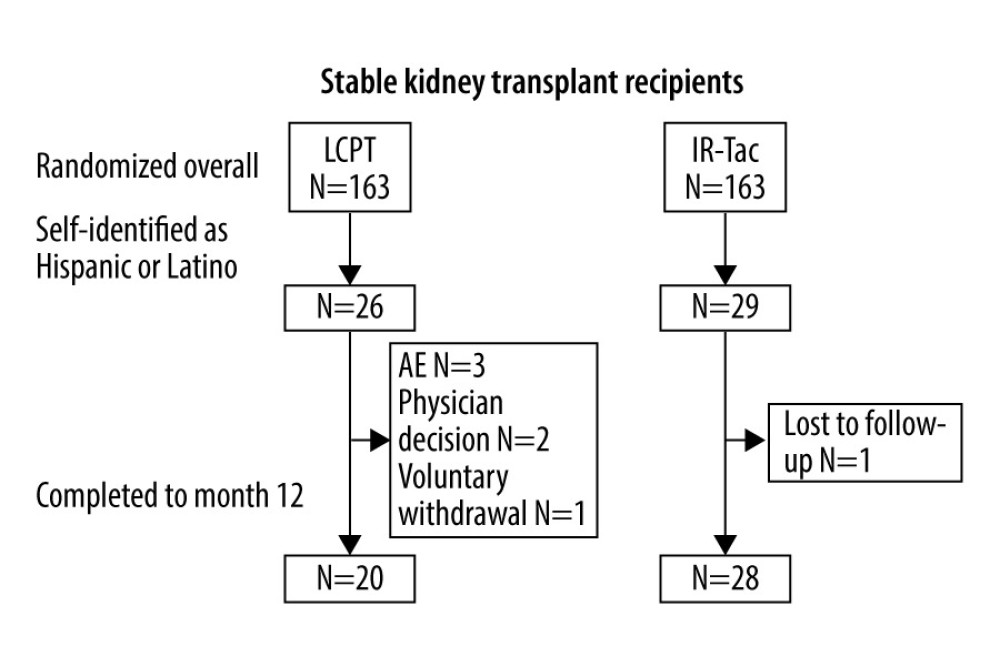 Figure 1. Patient disposition. AE – adverse event; IR-Tac – immediate-release tacrolimus; LCPT – LCP-tacrolimus.
Figure 1. Patient disposition. AE – adverse event; IR-Tac – immediate-release tacrolimus; LCPT – LCP-tacrolimus. 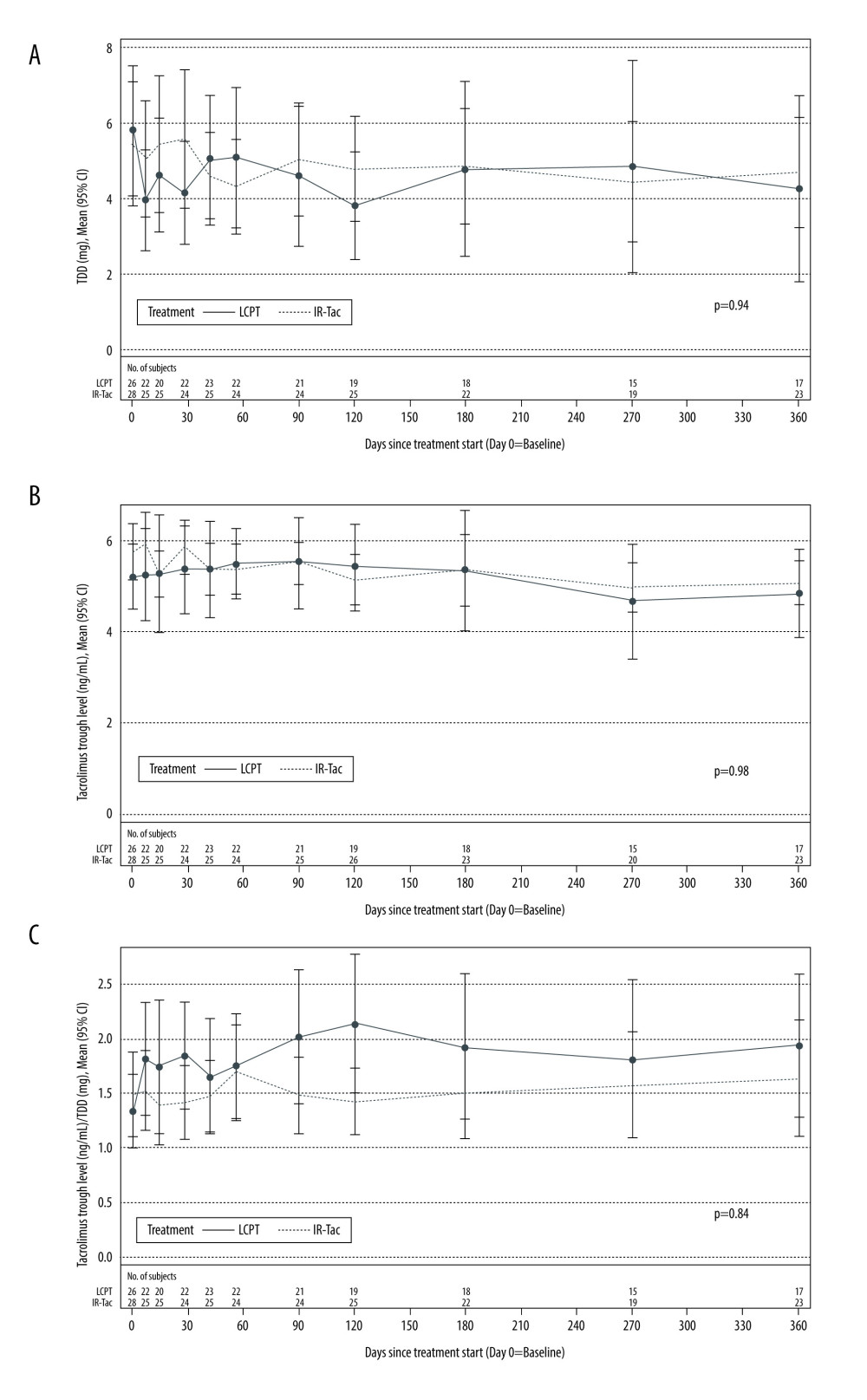 Figure 2. TDD (A), tacrolimus trough concentration (B), and the ratio of tacrolimus trough concentration to TDD (C) in Hispanic/Latino stable kidney transplant recipients. P values were calculated by general linear model for fixed effect of treatment×time. CI – confidence interval; IR-Tac – immediate-release tacrolimus; LCPT – LCP-tacrolimus; TDD – total daily dose.
Figure 2. TDD (A), tacrolimus trough concentration (B), and the ratio of tacrolimus trough concentration to TDD (C) in Hispanic/Latino stable kidney transplant recipients. P values were calculated by general linear model for fixed effect of treatment×time. CI – confidence interval; IR-Tac – immediate-release tacrolimus; LCPT – LCP-tacrolimus; TDD – total daily dose. 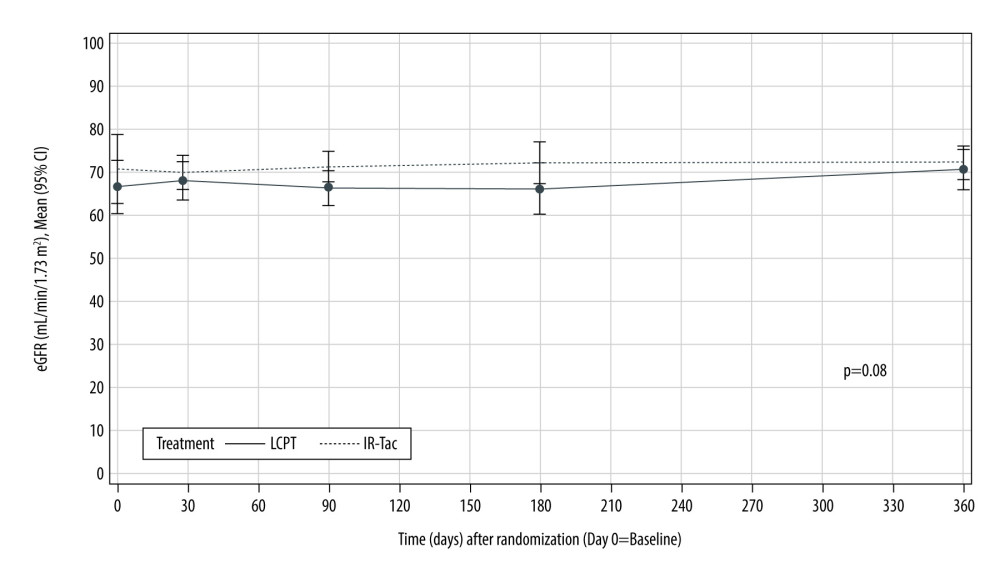 Figure 3. Renal function in Hispanic/Latino stable kidney transplant recipients. eGFR – estimated glomerular filtration rate; IR-Tac – immediate-release tacrolimus; LCPT – LCP-tacrolimus.
Figure 3. Renal function in Hispanic/Latino stable kidney transplant recipients. eGFR – estimated glomerular filtration rate; IR-Tac – immediate-release tacrolimus; LCPT – LCP-tacrolimus. Tables
Table 1. Within each study, no significant differences were detected between Hispanic/Latino patients in the LCPT and IR-Tac groups.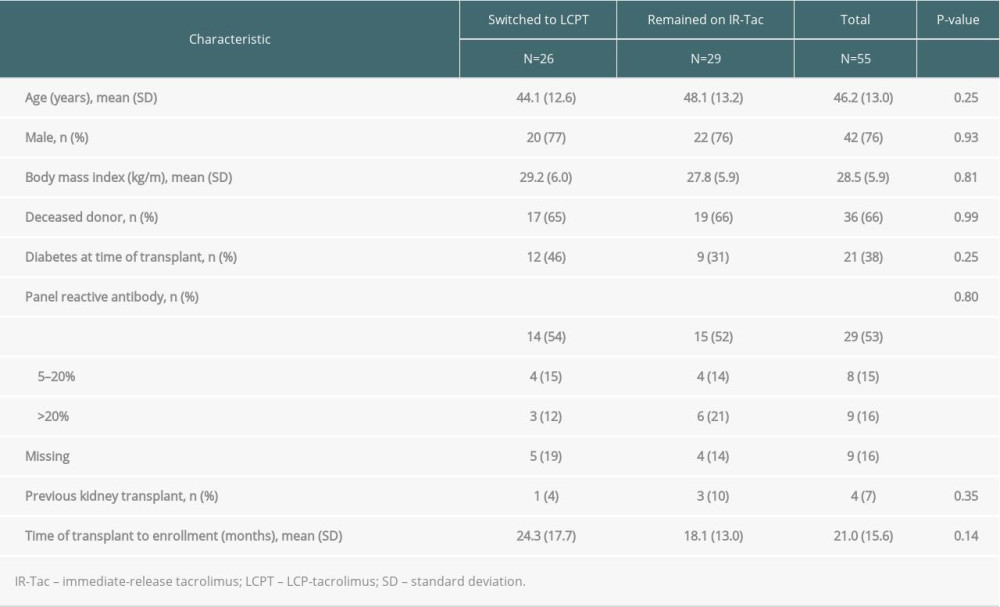 Table 2. Rates of treatment failure at 12 months.
Table 2. Rates of treatment failure at 12 months.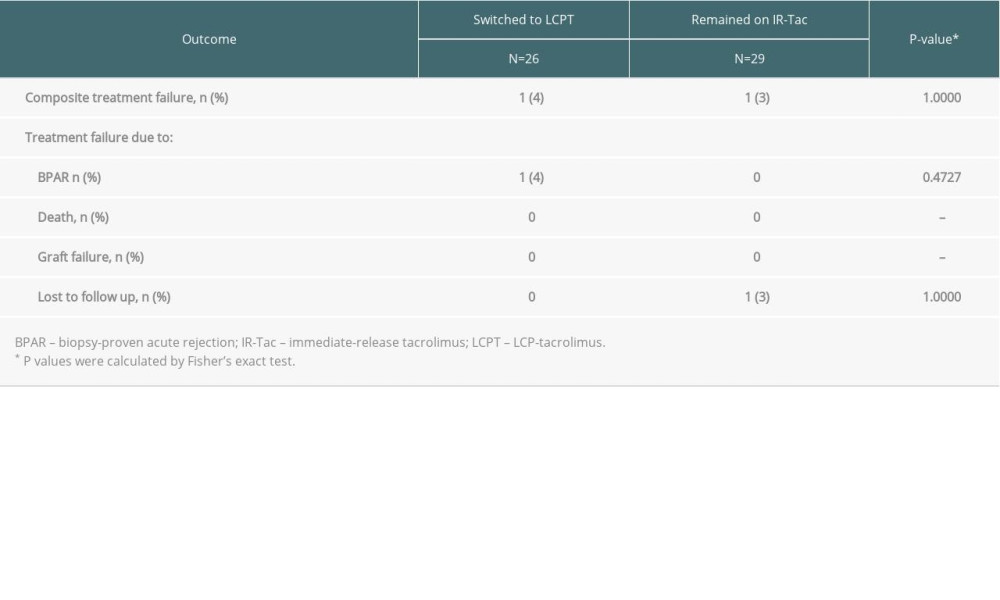 Table 3. Fisher’s exact test was performed to compare the proportion of patients who either did or did not experience the adverse event.
Table 3. Fisher’s exact test was performed to compare the proportion of patients who either did or did not experience the adverse event.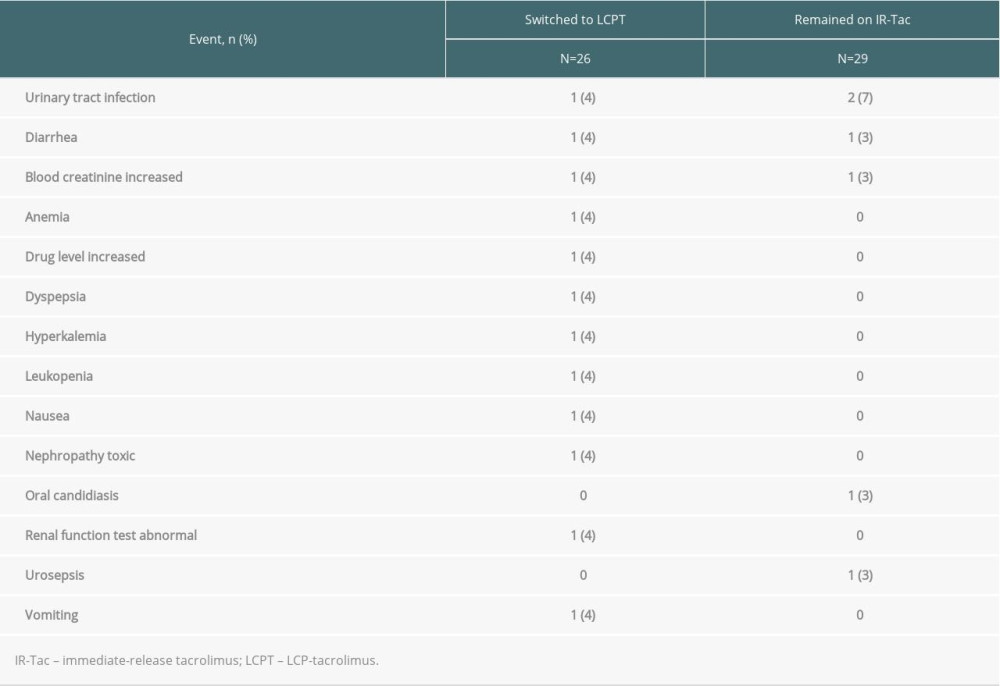
References
1. Oberbauer R, Bestard O, Furian L, Optimization of tacrolimus in kidney transplantation: New pharmacokinetic perspectives: Transplant Rev (Orlando), 2020; 34(2); 10053
2. US Food and Drugs Administration: Draft Guidance on Tacrolimus https://www.accessdata.fda.gov/drugsatfda_docs/psg/Tacrolimus_oral%20ER%20tablet_RLD%20206406_RC06-16.pdf
3. Brunet M, van Gelder T, Asberg A, Therapeutic drug monitoring of tacrolimus-personalized therapy: Second consensus report: Ther Drug Monit, 2019; 41(3); 261-307
4. Kamar N, Cassuto E, Piotti G, Pharmacokinetics of prolonged-release once-daily formulations of tacrolimus in de novo kidney transplant recipients: A randomized, parallel-group, open-label, multicenter study: Adv Ther, 2019; 36(2); 462-77
5. Sanchez Fructuoso A, Ruiz JC, Franco A, Effectiveness and safety of the conversion to MeltDose((R)) extended-release tacrolimus from other formulations of tacrolimus in stable kidney transplant patients: A retrospective study: Clin Transplant, 2020; 34(1); e13767
6. Taber DJ, Su Z, Fleming JN, Tacrolimus trough concentration variability and disparities in African American kidney transplantation: Transplantation, 2017; 101(12); 2931-38
7. Sanghavi K, Brundage RC, Miller MB, Genotype-guided tacrolimus dosing in African-American kidney transplant recipients: Pharmacogenomics J, 2017; 17(1); 61-68
8. Mohamed ME, Schladt DP, Guan W, Tacrolimus troughs and genetic determinants of metabolism in kidney transplant recipients: A comparison of four ancestry groups: Am J Transplant, 2019; 19(10); 2795-804
9. Neylan JF, Racial differences in renal transplantation after immunosuppression with tacrolimus versus cyclosporine: FK506 Kidney Transplant Study Group. Transplantation, 1998; 65(4); 515-23
10. Rostaing L, Bunnapradist S, Grinyo JM, Novel once-daily extended-release tacrolimus versus twice-daily tacrolimus in de novo kidney transplant recipients: Two-year results of phase 3, double-blind, randomized trial: Am J Kidney Dis, 2016; 67(4); 648-59
11. Bunnapradist S, Rostaing L, Alloway RR, LCPT once-daily extended-release tacrolimus tablets versus twice-daily capsules: A pooled analysis of two phase 3 trials in important de novo and stable kidney transplant recipient subgroups: Transpl Int, 2016; 29(5); 603-11
12. United States Census Bureau: Quick facts. United States https://www.census.gov/quickfacts/fact/table/US/RHI725218
13. National Institute of Diabetes and Digestive and Kidney Diseases: Kidney Disease Statistics for the United States https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease
14. Hart A, Smith JM, Skeans MA, OPTN/SRTR 2016 Annual Data Report: Kidney: Am J Transplant, 2018; 18(Suppl 1); 18-113
15. Claudio-Campos K, Duconge J, Cadilla CL, Ruano G, Pharmacogenetics of drug-metabolizing enzymes in US Hispanics: Drug Metab Pers Ther, 2015; 30(2); 87-105
16. Lamba J, Hebert JM, Schuetz EG, Klein TE, Altman RB, PharmGKB summary: Very important pharmacogene information for CYP3A5: Pharmacogenet Genomics, 2012; 22(7); 555-58
17. Rojas L, Neumann I, Herrero MJ, Effect of CYP3A5*3 on kidney transplant recipients treated with tacrolimus: A systematic review and meta-analysis of observational studies: Pharmacogenomics J, 2015; 15(1); 38-48
18. Khan AR, Raza A, Firasat S, Abid A, CYP3A5 gene polymorphisms and their impact on dosage and trough concentration of tacrolimus among kidney transplant patients: A systematic review and meta-analysis: Pharmacogenomics J, 2020; 20(4); 553-62
19. Ciancio G, Burke GW, Suzart K, The use of daclizumab, tacrolimus and mycophenolate mofetil in African-American and Hispanic first renal transplant recipients: Am J Transplant, 2003; 3(8); 1010-16
20. Eghtesad B, Malhotra D, Hecker WP, Use of tacrolimus as the primary immunosuppression after renal transplant in Native Americans and Hispanics: Transplant Proc, 1998; 30(4); 1232-33
21. García-Roca P, Medeiros M, Reyes H, CYP3A5 polymorphism in Mexican renal transplant recipients and its association with tacrolimus dosing: Arch Med Res, 2012; 43(4); 283-87
22. Mancinelli LM, Frassetto L, Floren LC, The pharmacokinetics and metabolic disposition of tacrolimus: A comparison across ethnic groups: Clin Pharmacol Ther, 2001; 69(1); 24-31
23. Bunnapradist S, Ciechanowski K, West-Thielke P, Conversion from twice-daily tacrolimus to once-daily extended release tacrolimus (LCPT): The phase III randomized MELT trial: Am J Transplant, 2013; 13(3); 760-69
24. Solez K, Colvin RB, Racusen LC, Banff 07 classification of renal allograft pathology: Updates and future directions: Am J Transplant, 2008; 8(4); 753-60
25. Cockcroft DW, Gault MH, Prediction of creatinine clearance from serum creatinine: Nephron, 1976; 16(1); 31-41
26. Nankivell BJ, Gruenewald SM, Allen RD, Chapman JR, Predicting glomerular filtration rate after kidney transplantation: Transplantation, 1995; 59(12); 1683-89
27. van Hooff JP, Christiaans MH, van Duijnhoven EM, Tacrolimus and posttransplant diabetes mellitus in renal transplantation: Transplantation, 2005; 79(11); 1465-69
28. Knops N, Levtchenko E, van den Heuvel B, Kuypers D, From gut to kidney: transporting and metabolizing calcineurin-inhibitors in solid organ transplantation: Int J Pharm, 2013; 452(1–2); 14-35
Figures
 Figure 1. Patient disposition. AE – adverse event; IR-Tac – immediate-release tacrolimus; LCPT – LCP-tacrolimus.
Figure 1. Patient disposition. AE – adverse event; IR-Tac – immediate-release tacrolimus; LCPT – LCP-tacrolimus. Figure 2. TDD (A), tacrolimus trough concentration (B), and the ratio of tacrolimus trough concentration to TDD (C) in Hispanic/Latino stable kidney transplant recipients. P values were calculated by general linear model for fixed effect of treatment×time. CI – confidence interval; IR-Tac – immediate-release tacrolimus; LCPT – LCP-tacrolimus; TDD – total daily dose.
Figure 2. TDD (A), tacrolimus trough concentration (B), and the ratio of tacrolimus trough concentration to TDD (C) in Hispanic/Latino stable kidney transplant recipients. P values were calculated by general linear model for fixed effect of treatment×time. CI – confidence interval; IR-Tac – immediate-release tacrolimus; LCPT – LCP-tacrolimus; TDD – total daily dose. Figure 3. Renal function in Hispanic/Latino stable kidney transplant recipients. eGFR – estimated glomerular filtration rate; IR-Tac – immediate-release tacrolimus; LCPT – LCP-tacrolimus.
Figure 3. Renal function in Hispanic/Latino stable kidney transplant recipients. eGFR – estimated glomerular filtration rate; IR-Tac – immediate-release tacrolimus; LCPT – LCP-tacrolimus. Tables
 Table 1. Within each study, no significant differences were detected between Hispanic/Latino patients in the LCPT and IR-Tac groups.
Table 1. Within each study, no significant differences were detected between Hispanic/Latino patients in the LCPT and IR-Tac groups. Table 2. Rates of treatment failure at 12 months.
Table 2. Rates of treatment failure at 12 months. Table 3. Fisher’s exact test was performed to compare the proportion of patients who either did or did not experience the adverse event.
Table 3. Fisher’s exact test was performed to compare the proportion of patients who either did or did not experience the adverse event. Table 1. Within each study, no significant differences were detected between Hispanic/Latino patients in the LCPT and IR-Tac groups.
Table 1. Within each study, no significant differences were detected between Hispanic/Latino patients in the LCPT and IR-Tac groups. Table 2. Rates of treatment failure at 12 months.
Table 2. Rates of treatment failure at 12 months. Table 3. Fisher’s exact test was performed to compare the proportion of patients who either did or did not experience the adverse event.
Table 3. Fisher’s exact test was performed to compare the proportion of patients who either did or did not experience the adverse event. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








