16 July 2021: Original Paper
Risk Factors Influencing the Outcomes of Kidney Re-Transplantation
Anke Schwarz1ADEF*, Frank Schäfer1AB, Theodor Framke2CD, Silvia Linnenweber-Held1AB, Nicolas Richter3BD, Hermann Haller1DEDOI: 10.12659/AOT.928922
Ann Transplant 2021; 26:e928922
Abstract
BACKGROUND: Our kidney transplant waitlist includes 20% re-transplantations (TX2). Knowing what to expect is a clinical obligation.
MATERIAL AND METHODS: We compared graft and patient survival of all 162 TX2 patients, transplanted 2000 to 2009, with 162 patients after first transplantation (TX1) matched for age, sex, living/non-living donation, and transplantation date. Patient follow-up was 10 years.
RESULTS: TX2 graft and patient survivals were inferior to TX1 (p<0.001 and p=0.047). TX2 patients had a longer cumulative dialysis vintage, more human leucocyte antigen (HLA) mismatches, more panel-reactive HLA antibodies, more often received induction therapy with rabbit-antithymocyte globulin (rATG), and had a lower body mass index (all p<0.05). Death from infection and graft failure by rejection was more frequent after TX2 (both p<0.05) but not after TX1. Multivariable Cox regression analysis revealed that both cohorts had graft failure and death risk associated with infection and cardiovascular disease, and graft failure by humoral rejection. However, only TX2 patients had an additional risk of graft failure with early inferior graft function and of patient death with ≥2 comorbidities. Moreover, Kaplan-Meier analysis showed that TX2 and not TX1 patients had a lower graft and patient survival associated with infection and with ≥2 comorbidities (all p<0.05).
CONCLUSIONS: Re-transplantation is associated with worse graft outcomes mainly because of immunologic and graft-quality reasons, although the high number of comorbidities and infection severities aside from cardiovascular disease drive mortality. The more frequent rATG induction of TX2 patients could promote infection by enhancing immunosuppression. By addressing comorbidities, outcomes could possibly be improved.
Keywords: renal insufficiency, Mortality, Reoperation, Waiting Lists, Kidney Transplantation, frailty, Graft Rejection, Kidney, Risk Factors
Background
Shortly after kidney transplantation was introduced, graft failure commonly meant patient death, since no kidney replacement therapy existed. Thus, to save lives, re-transplantation (TX2) was introduced in 1963; however, at that time, the 1-year patient survival was only 60% [1]. The Hannover Medical School transplant program is one of the largest in Germany, with 654 patients on the active waiting list (February 2020). Of these, 123 patients (19%) have been transplanted before and 22 (4%) more than once. In Germany, the interminable waiting list makes matters even more acute [2,3]. We sought to learn the particular hazards faced by re-transplanted patients. Repeat transplantation has been the subject of numerous publications since 1974, demonstrating increasingly better results in graft and patient survival [4–11]. In some reports, hardly any difference in graft or patient survival was found [10,11]. However, currently the results are conflicting and sometimes difficult to interpret. We conducted a retrospective analysis of re-transplanted patients compared to a matched control group of patients with first transplantation to identify particular risk factors for graft and patient survival, so that inherent problems might be addressed prospectively in future studies.
Material and Methods
STATISTICAL ANALYSIS:
We performed a retrospective, single-center, matched-pair investigation. Matching was performed on a 1: 1 basis where patients with a second transplantation served as cases (TX2 group) and patients who received their first transplant during the same period served as controls (TX1 group). Matching criteria were recipient age (±10 years), sex, living/non-living kidney donation, and transplantation date (±18 months). Descriptive analysis of the data included presenting relative and absolute frequencies for categorical data. Continuous variables are presented as arithmetic mean and standard deviation. Baseline characteristics of cases and controls were compared with a paired t test for continuous variables and McNemar’s test for binary outcomes. A time-to-event analysis was performed for cases and controls separately. Graft survival or patient survival served as an event. Times were censored for graft survival at the date of the last hospital visit if a patient was lost to follow-up or had a functioning graft. For patient survival, times were censored at the last hospital visit if a patient was lost to follow-up or had a functioning graft or a transplant failure. Kaplan-Meier curves were plotted for cases (TX2) and controls (TX1) separately. A log-rank test and a Cox regression model were used to compare relevant covariables. To account for the matched-pair design, a marginal Cox regression model was set up to compare covariables as well as cases and controls. To compare graft and patient survival between TX1 and TX2, we used a test as described in Klein and Moeschberger [12]. Multivariable Cox regression models are presented for a set of 8 covariables that were deemed of high relevance. The full model is presented as well as models after variable selection. We applied several approaches (backward selection, score-based best subset selection, and stepwise selection). All analyses were done with SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
KAPLAN-MEIER CURVES:
For the variables ≥50 or <50 years of age, HLA mismatches, initial graft function, post-transplant serum creatinine at hospital discharge, humoral rejection, cardiovascular disease, number of comorbidities, and severe infection, differences of the clinical course in graft and/or patient survival were tested by Kaplan-Meier curves. TX2 patients had a lower graft and patient survival associated with vs without severe infection (Figure 2) and with 3–5 vs 0–2 comorbidities (Figure 3). Graft and patient survival were also lower in TX2 patients with 4–6 vs 0–3 HLA mismatches (log-rank p=0.015 and 0.004, respectively). TX2 patients had a lower graft survival with serum creatinine ≥150 vs <150μmol/L at hospital discharge (Supplementary Figure 1, log-rank p=0.003). Despite the same number of humoral rejections, graft survival only of TX2 patients was lower with vs without humoral rejection (Supplementary Figure 2, log-rank p=0.013). Both cohorts had a lower graft and patient survival associated with vs without cardiovascular disease (Figure 4), as well as a lower patient survival in patients ≥50 vs <50 years of age (log-rank TX2 p=0.018 and TX1 p=0.027), and in patients with malignancy (log-rank TX2 p=0.038 and TX1 p<0.001). Both cohorts had a higher graft survival with initial graft function (log-rank TX2 p=0.002 and TX1 0.038).
COX REGRESSION ANALYSIS:
Regarding graft and patient survival, higher and lower hazard ratios (HR) for the different covariables are enumerated in Supplementary Tables 1 and 2.
Multivariable analysis for graft survival showed that only TX2 and not TX1 patients had a higher risk associated with early inferior graft function; while both cohorts had a higher risk together with cardiovascular disease, severe infection, and humoral rejection (Table 3A). Patient survival showed that only TX2 and not TX1 patients had a higher risk for death together with a high number of comorbidities, while both cohorts had a higher death risk together with cardiovascular disease and severe infection (Table 3B).
Discussion
We found that graft and patient survival in TX2 patients were inferior to TX1 patients (Figure 1). There were numerous explanations in terms of pre-transplant conditions. TX2 patients were confronted with longer times on dialysis, they had a greater immunological risk by their higher immunization rate, and had more HLA mismatches by avoidance of HLA-matches related to their high PRAs (Table 1). Thus, TX2 patients were more likely to receive rATG induction therapy (Table 1).
Inspecting outcomes in detail, TX2 patients generally had lower rates of graft function, more commonly suffered graft loss from rejection, and more often died of severe infection (Table 2). Multivariable Cox regression models for 8 important covariables showed that fundamentally both cohorts had some similar problems with graft and patient survival, namely a risk associated with severe infection and cardiovascular disease (Table 3A, 3B). However, TX2 and not TX1 patients additionally had a lower graft survival with early restricted graft function (Supplementary Figure 1) and had a higher death risk with a high number of comorbidities (Table 3B). This was confirmed in Kaplan-Meier analysis. In patients with cardiovascular disease, the course of graft and patient survival was inferior in both cohorts (Figure 4). However, in TX2 patients, this relationship was more robust, as seen in the distinctly inferior Kaplan-Meier curve of the TX2 cohort (Figure 4A, 4B). The same is true for patient survival in patients with a high number of comorbidities and a distinctly inferior Kaplan-Meier curve in TX2 patients (Figure 3A, 3B).
In the TX2 group, most infections were in the early transplant period (≤6 months) compared to the TX1 group (Table 4, Figure 4A). This state of affairs at least partially may be due to the more frequent use of rATG induction therapy. The TX2 group had a higher immunization rate, and patients were treated accordingly (Table 1). Rabbit ATG is very efficacious in the prophylaxis of rejection episodes in patients at risk of rejection [13,14]. T-cell or T-cell subset suppression even in low-dose treatment of rATG lasts 6 to 12 months until slight recovery [15,16]. It is not clear how long the propensity to infection lasts after the use of rATG, and the timely coincidence of rATG and severe infection during the first 6 months after transplantation is only an indirect sign of a possibly causal role of rATG [14,17].
Transplant nephrectomy of the preceding failed transplant was not an advantage regarding rejection in the second graft, which confirms recent studies [18,19]. TX2 patients had a distinctly lower BMI than TX1 patients (Table 1 and Table 3A, 3B), which has been reported before [6,11]. This finding may be due to the longer cumulative dialysis vintage (Table 1 and Table 3A, 3B) resulting in weight loss associated with mortality [20,21]. However, compared to dialysis, after transplantation we did not find a low BMI correlating to mortality, but within both cohorts we found an improved patient survival at least tested against a BMI above the limit of overweight and obesity (≥25; Table 3B). For obese transplant patients, in general an inferior graft and patient survival, compared to non-obese patients, has been reported [6,22,23].
We did not find an influence of the longer dialysis vintage on graft survival of the TX2 compared to the TX1 group (Table 1 and Table 3A, 3B). Indeed, in former publications such an impact has been convincingly reported and was regarded as one of the main negative influences on graft survival [24]. However, in more recent publications this was no longer found. This can be attributed in general to the better dialysis conditions and especially to the reduced need for blood transfusions because of the regular supply of the dialysis patients with erythropoietin and iron; thus, a main source of pre-transplant immunization has reduced [25,26].
Another reason for a continuously better graft survival of repeat transplantation is the development of better techniques of HLA class II typing as well as HLA-antibody detection [27–32]. Complement-dependent crossmatch had for a long time been the only method to prevent hyperacute rejection and to test for HLA antibodies [33,34]. HLA typing and antibody detection has improved the second graft survival rate since 1974 [1,5,9–11]. However, all-cause mortality is still influenced by a longer dialysis vintage [25,26,35]. We did not find a direct association between duration of dialysis vintage and mortality, but we saw that TX2 patients had a higher mortality caused by severe infection (Tables 2, 4), associated with a high number of comorbidities, and with cardiovascular disease (Figures 3, 4). Thus, the higher morbidity of TX2 patients causing mortality can be interpreted as an indirect consequence of the longer dialysis vintage as one of the basal conditions causing this morbidity.
We compare our results with those reported earlier. Before 2005, TX2 patients generally had worse outcomes except under selected favorable subgroup conditions [4,24]. However, these cohorts were usually not well defined and living-donor status and cyclosporine treatment were not always mentioned. Since 2007, the cohorts of second and first transplantation were mostly compared studying large-registry databases [5,6,7,9,10]. Since then, the transplant outcome gap between both cohorts has constantly narrowed but did not become similar. Registry data, with their multicenter sources, different modes of access to transplantation, and different immunosuppressive treatments, cannot be easily compared to single-center studies. A strength of the present study is the fact that we compared 2 rather uniform cohorts transplanted in a limited similar 10-year timeframe under controlled conditions by the same team and with a follow-up period of 10 years after the end of the study. TX2 results are inferior to TX1-matched controls (Figure 1). This finding contradicts a large single-center study of 3000 patients recently published [11]. However, in that study, living donation was common (71% of TX2 patients), which makes the study less comparable to ours. We were interested to learn that 2 or more re-transplantations have no worse graft and patient survival than 1 re-transplantation [36].
The factors influencing poorer graft and patient outcome may represent a more general inability of overcoming unfavorable conditions of the post-transplant course. This state of affairs brings to mind impaired resistance in TX2, compared to TX1 patients, a condition also called frailty. Frailty has been described and standardized in older patients as indicating physical and cognitive pre-aging and has been related to decreased physiologic reserve and resistance to stressors [37]. Chronic kidney disease has been found to be associated with a higher frailty score, increasing with the stage of renal insufficiency [38,39]. After renal transplantation, frail patients have a higher risk of death [40]. Here, we did not measure physical and/or cognitive parameters, which was not possible because of the retrospective character of the analysis. However, loss of body weight and a lower BMI, as observed in TX2 patients (Table 1 and Table 3A, 3B) are main features of frailty [37]. Conceivably, DNA methylation could be measured in such patients [41], and epigenetic age acceleration has been found to be correlated to other physical and cognitive frailty parameters. Nevertheless, this has mostly been evaluated in large cohorts [42].
The poorer performance of repeat transplantation is related to several factors regarding immunologic, graft-quality, and infectious problems, of which the most important for patient survival were a high number of comorbidities and severity of infections (Table 3A, 3B, Figures 2–4). Immunologic sensitization together with more HLA mismatches contributes (Table 1). This situation more often implies a higher immunosuppressive induction therapy, as was the rule in the TX2 patient cohort by rATG (Table 1), favoring early post-transplant infections. The combination of a higher immunological risk and a possibly generally higher underlying frailty of the TX2 group may be the causal factors for the inferior graft and patient survival of the TX2 patients compared to matched patients with a first transplant. Dealing with comorbidities in any clinical setting is not trivial. In this population, the task is daunting. Nevertheless, our data underscore that this state of affairs could be addressed with diagnostic controls as well as appropriate therapies.
The alternative to repeat transplantation is dialysis. As known from the literature, first transplantation as well as re-transplantation are both clearly better than dialysis; therefore, even preemptive second transplantation has been proposed [43–45]. The relative risk reduction by transplantation seems to be higher in re-transplanted patients because of their higher mortality on the waiting list [10]. To avoid that fate, these higher-risk patients deserve all the chances we can provide by repeated transplantation [46,47].
Conclusions
TX2 graft and patient survivals are inferior to TX1. However, we have not only to compare TX2 to TX1 but also compare them to dialysis patients as the alternative treatment option, and dialysis would mean an even higher mortality. The higher number of comorbidities is, beside immunologic and infectious problems, the main risk factor for inferior outcomes of TX2 patients. Therefore, we should try as far as possible to address comorbidities by preventing and treating them.
Figures
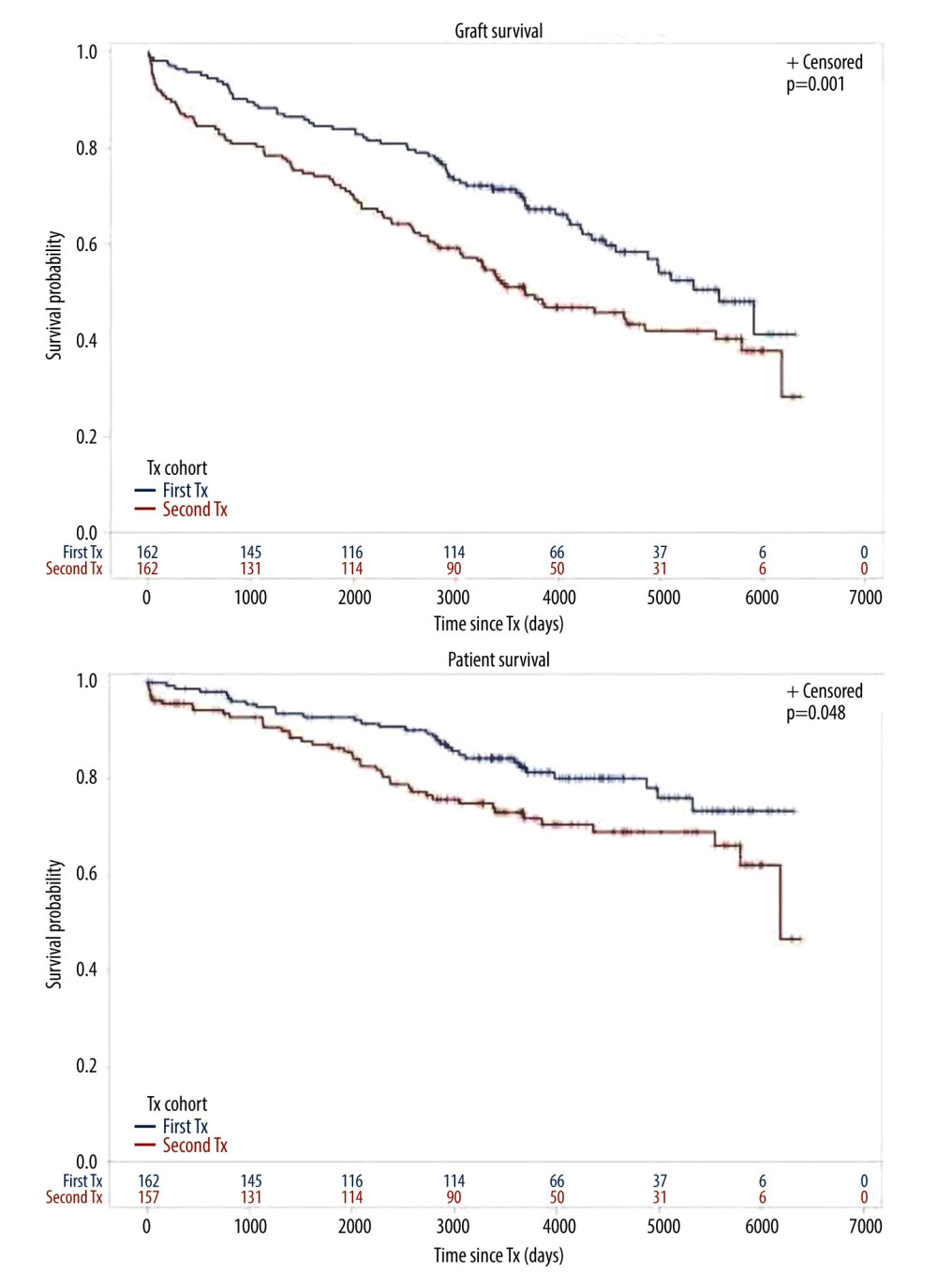 Figure 1. Cohort TX2 (162 patients after kidney re-transplantation) compared to TX1 (162 matched control patients after first transplantation)Graft survival (p<0.001), as well as patient survival (0.048) were significantly inferior in TX2 compared to TX1 patients.
Figure 1. Cohort TX2 (162 patients after kidney re-transplantation) compared to TX1 (162 matched control patients after first transplantation)Graft survival (p<0.001), as well as patient survival (0.048) were significantly inferior in TX2 compared to TX1 patients. 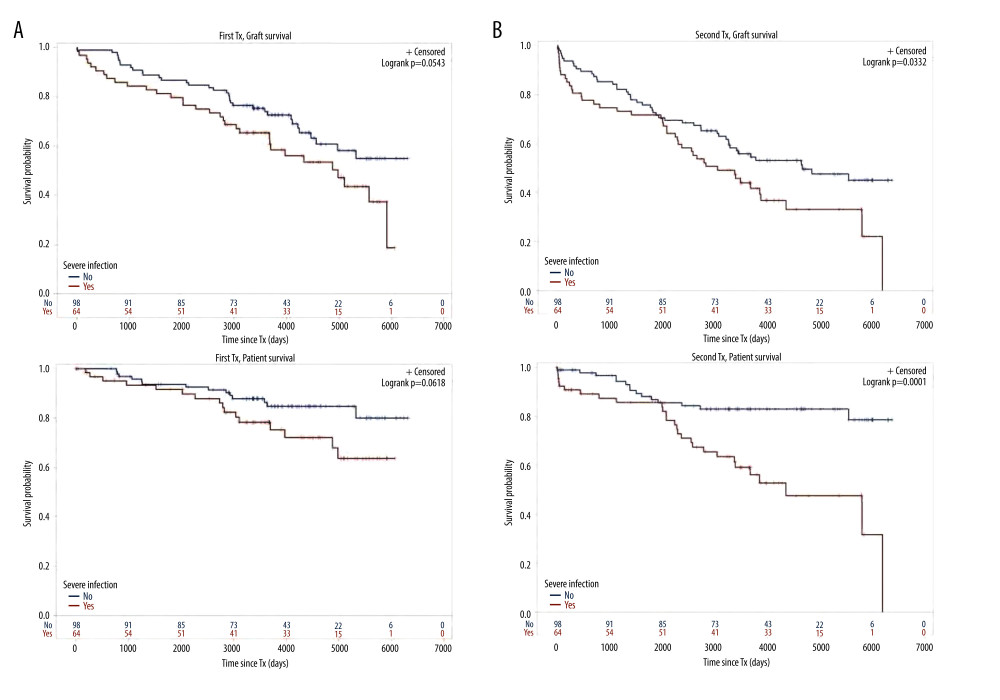 Figure 2. Severe infection endangering graft and/or patient survival(A) Graft and patient survival of 64 TX1 patients with severe infection compared to 98 TX1 patients without were not significantly different. (B) However, graft and patient survival of 67 TX2 patients with severe infection compared to 95 TX2 patients without were significantly inferior (p=0.0332 and p=0.0001, respectively).
Figure 2. Severe infection endangering graft and/or patient survival(A) Graft and patient survival of 64 TX1 patients with severe infection compared to 98 TX1 patients without were not significantly different. (B) However, graft and patient survival of 67 TX2 patients with severe infection compared to 95 TX2 patients without were significantly inferior (p=0.0332 and p=0.0001, respectively). 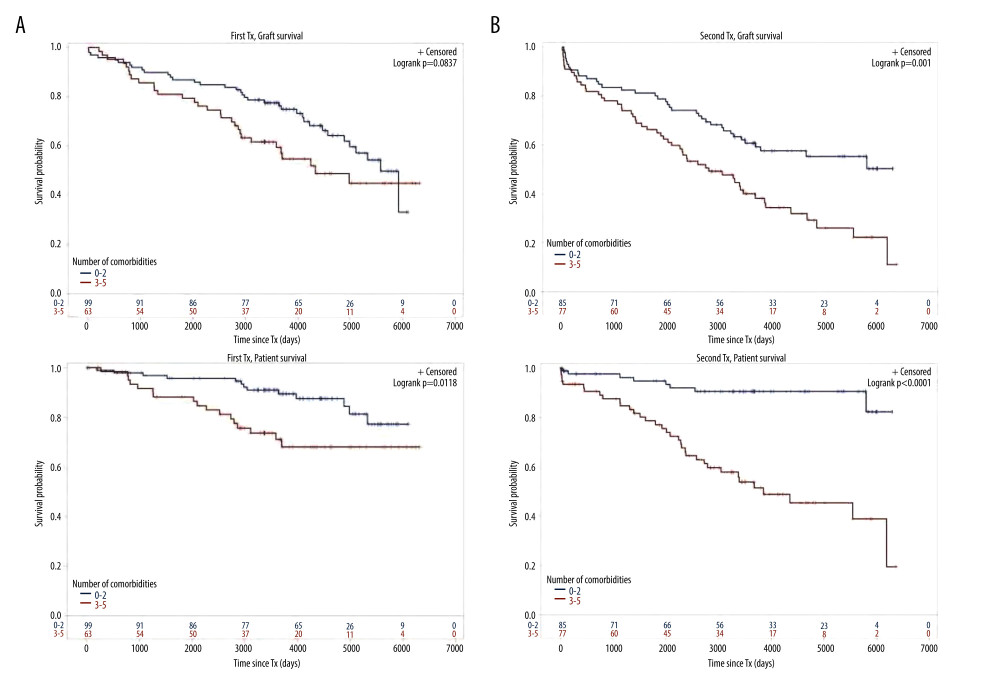 Figure 3. Number of comorbidities(A) Graft survival of 63 TX1 patients with 3–5 compared to 99 TX1 patients with 0–2 comorbidities was not statistically different; while patient survival of TX1 patients with 3–5 comorbidities, was significantly inferior to those with 0–2 (p=0.0118). (B) Graft as well as patient survival of 77 TX2 patients with 3–5 compared to 85 TX2 patients with 0–2 comorbidities were significantly inferior (p=0.001 and p<0.0001 respectively).
Figure 3. Number of comorbidities(A) Graft survival of 63 TX1 patients with 3–5 compared to 99 TX1 patients with 0–2 comorbidities was not statistically different; while patient survival of TX1 patients with 3–5 comorbidities, was significantly inferior to those with 0–2 (p=0.0118). (B) Graft as well as patient survival of 77 TX2 patients with 3–5 compared to 85 TX2 patients with 0–2 comorbidities were significantly inferior (p=0.001 and p<0.0001 respectively). 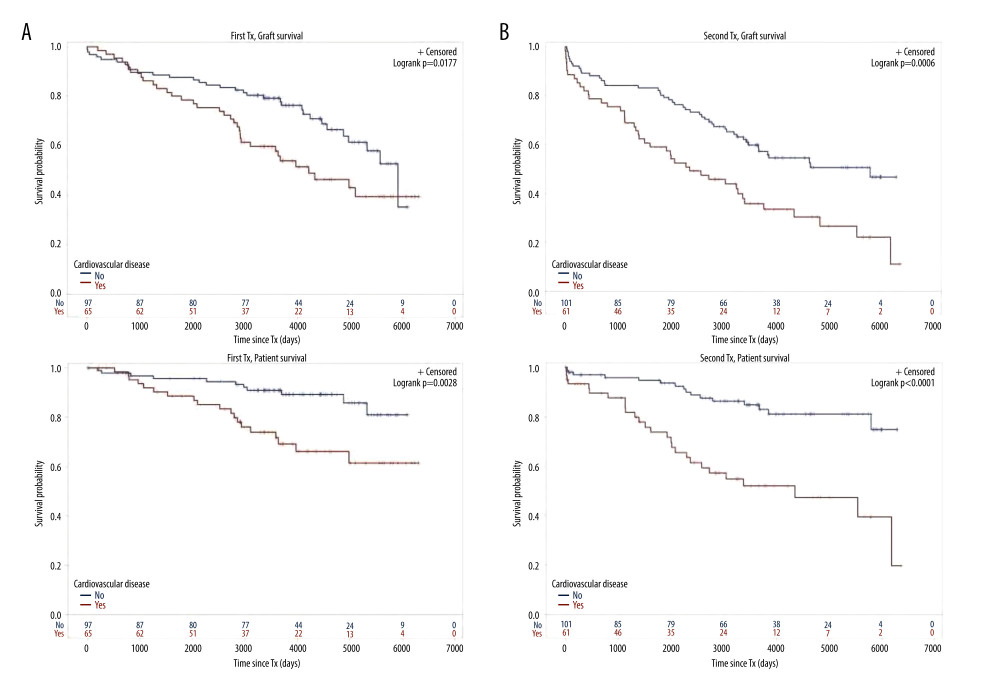 Figure 4. Cardiovascular disease(A) Graft as well as patient survival of 65 TX1 patients with compared to 97 TX1 patients without cardiovascular disease were significantly inferior (p=0.0177 and p=0. 0028, respectively). (B) Graft as well as patient survival in 61 TX2 patients with compared to 101 TX2 patients without cardiovascular disease were significantly inferior. However, in TX2 patients this relationship was more robust than in TX1 patients (p=0.0006 and p=p<0.0001, respectively).
Figure 4. Cardiovascular disease(A) Graft as well as patient survival of 65 TX1 patients with compared to 97 TX1 patients without cardiovascular disease were significantly inferior (p=0.0177 and p=0. 0028, respectively). (B) Graft as well as patient survival in 61 TX2 patients with compared to 101 TX2 patients without cardiovascular disease were significantly inferior. However, in TX2 patients this relationship was more robust than in TX1 patients (p=0.0006 and p=p<0.0001, respectively). Tables
Table 1. Demographic data of group TX1 (first transplantation) and TX2 (re-transplantation).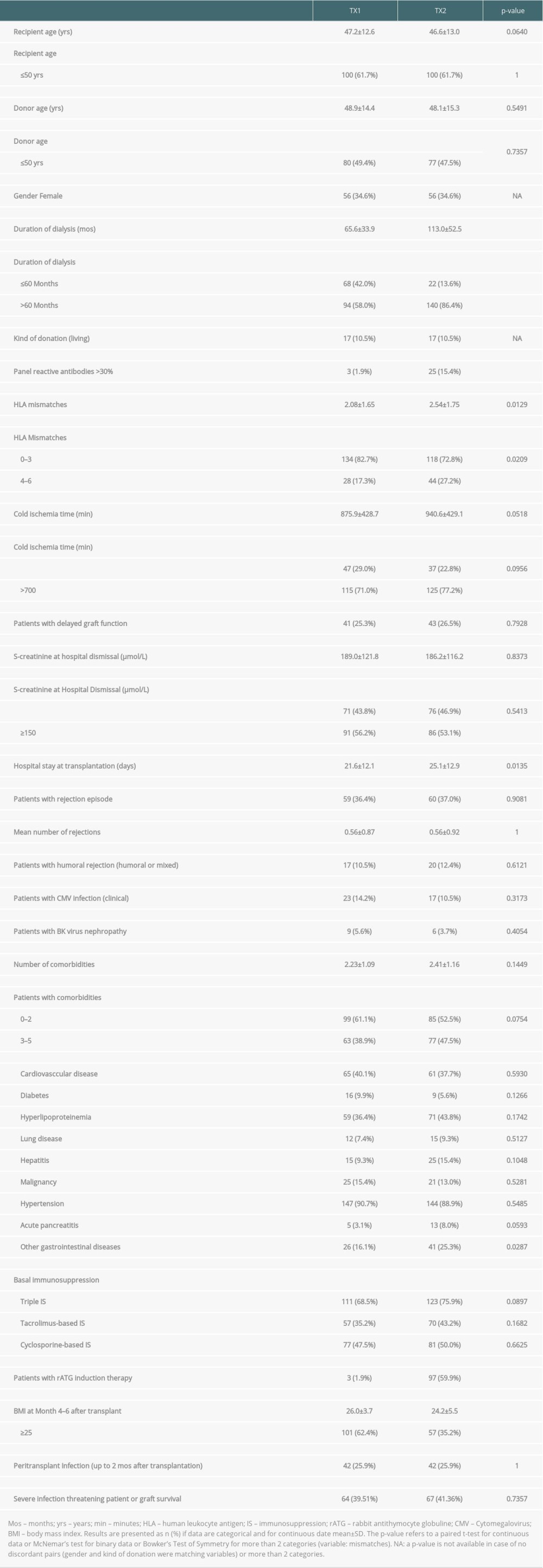 Table 2. Outcome of patient group TX2 (re-transplantation) compared to TX1 (first transplantation) serving as control.
Table 2. Outcome of patient group TX2 (re-transplantation) compared to TX1 (first transplantation) serving as control.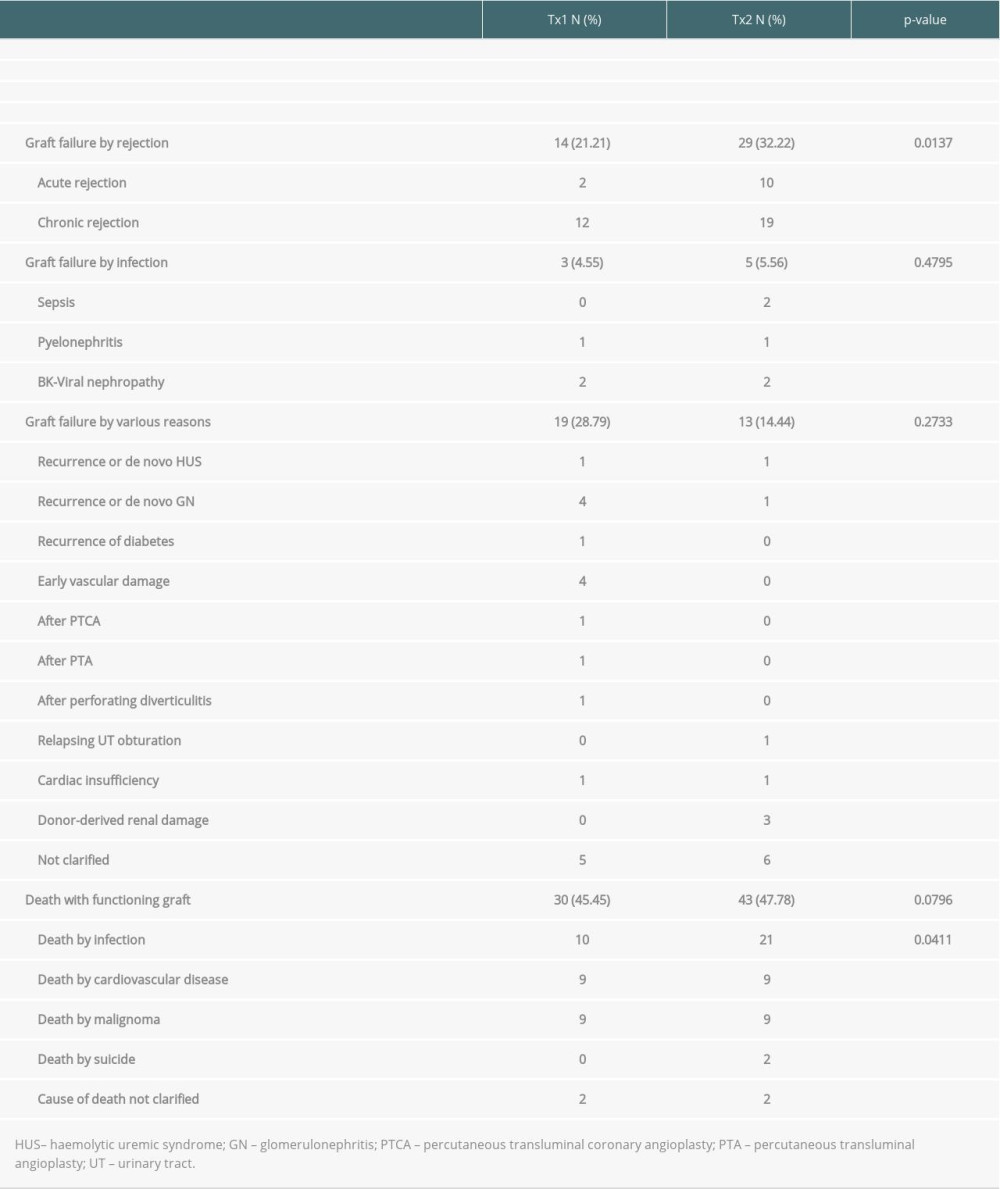 Table 3A. Time-to-event analysis of graft survival (multivariable model and variable selection).
Table 3A. Time-to-event analysis of graft survival (multivariable model and variable selection).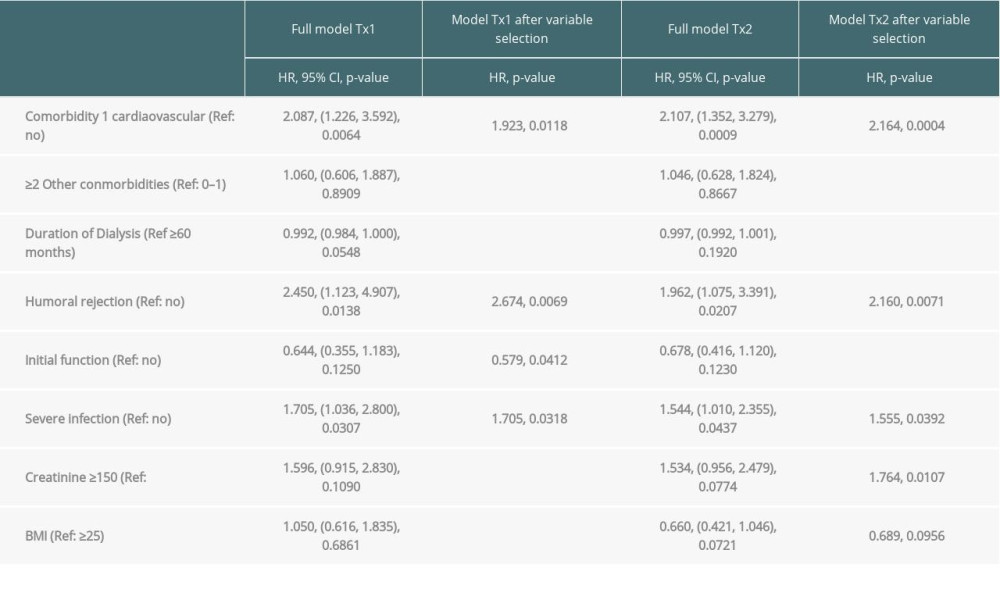 Table 3B. Time-to-event analysis of patient survival (multivariable model and variable selection).
Table 3B. Time-to-event analysis of patient survival (multivariable model and variable selection).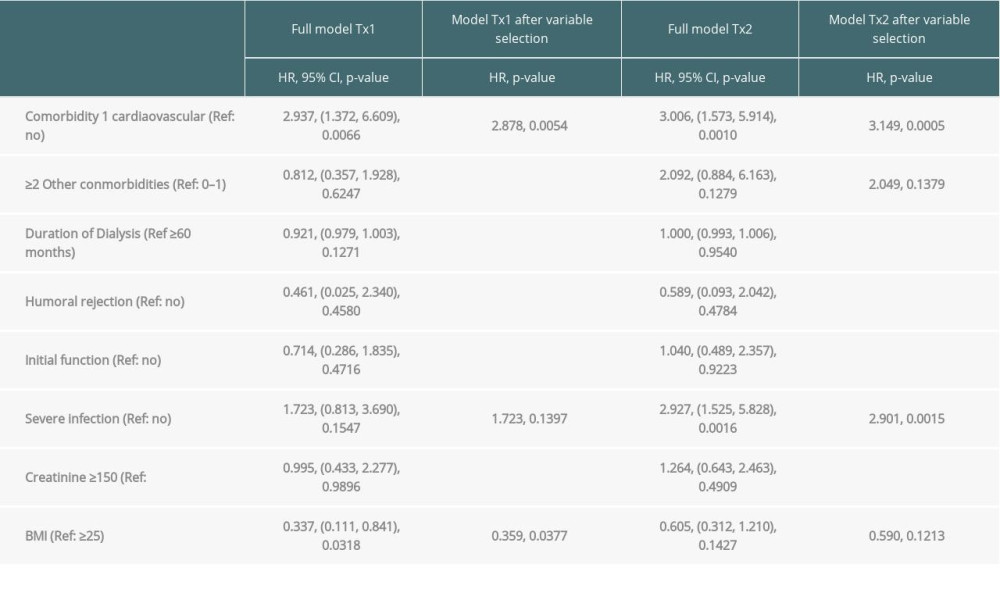 Table 4. Severe infections threatening life or graft survival.
Table 4. Severe infections threatening life or graft survival.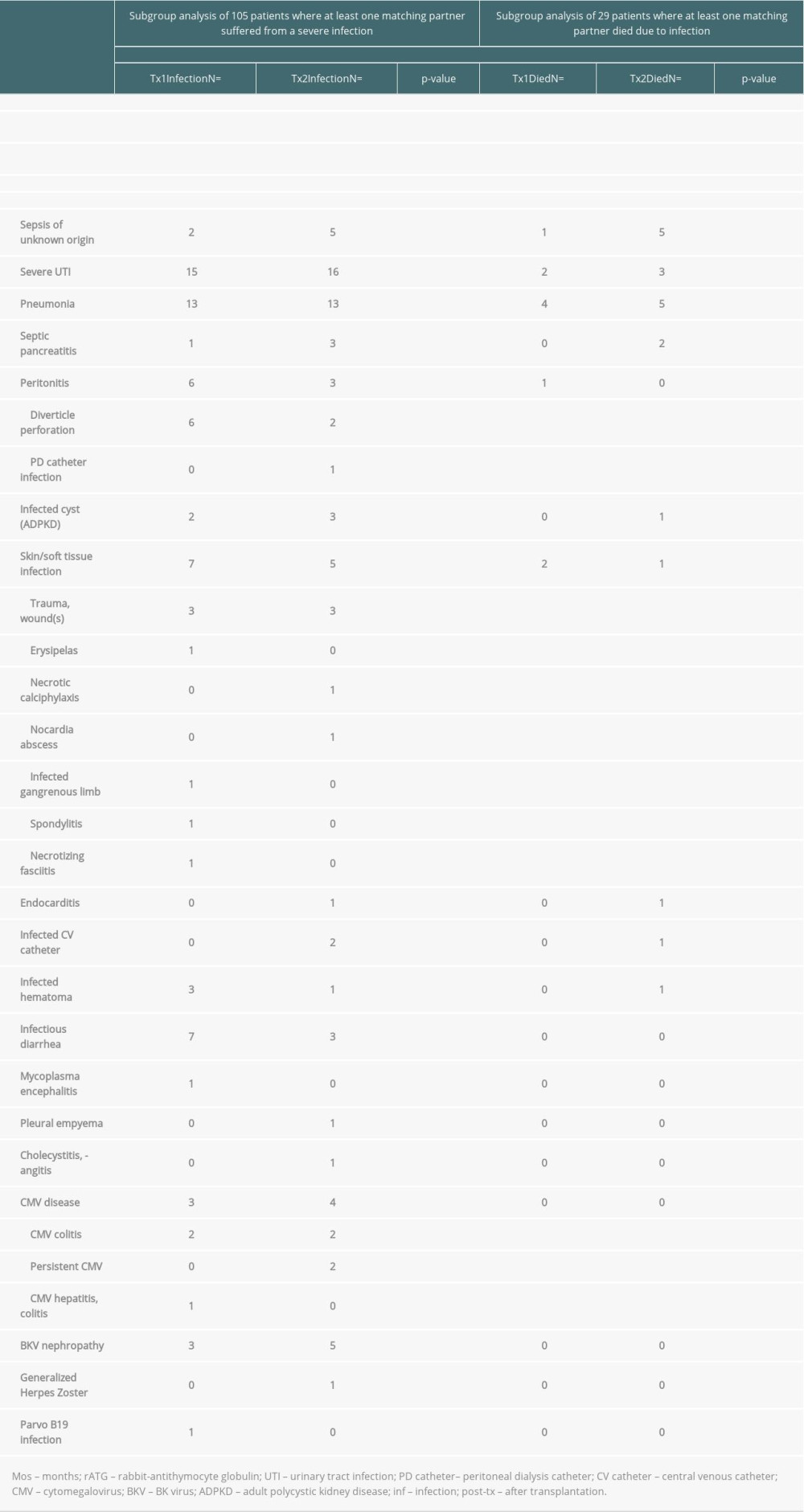 Supplementary Table 1. Graft survival (defined as time to transplant failure or death); time-to-event analysis of graft survival (univariate).
Supplementary Table 1. Graft survival (defined as time to transplant failure or death); time-to-event analysis of graft survival (univariate).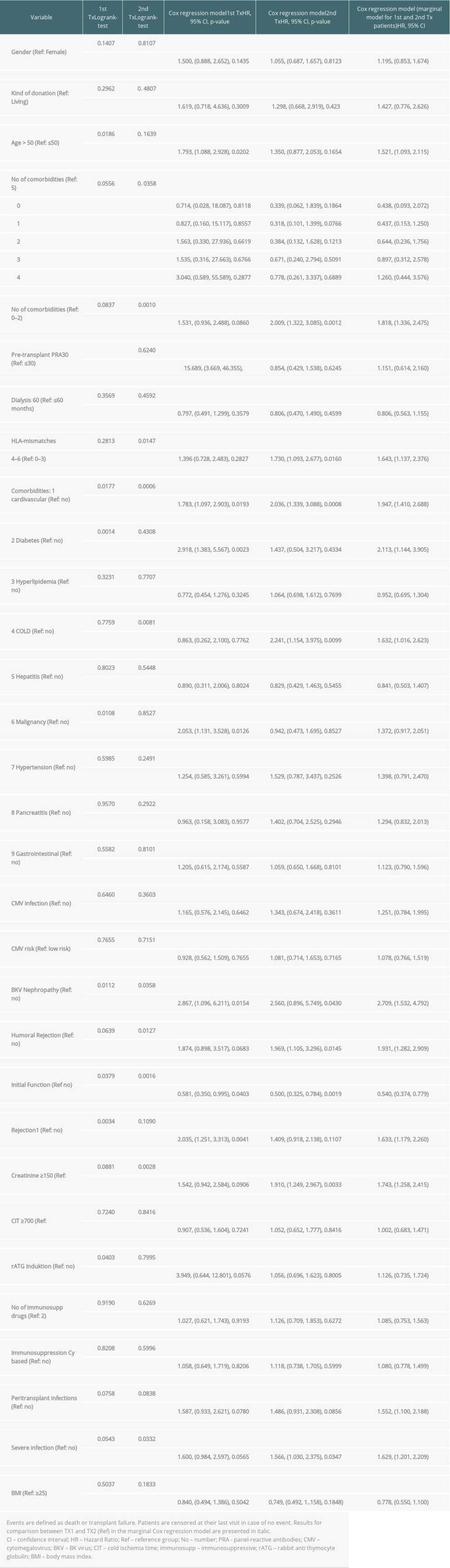 Supplementary Table 2. Patient survival (defined as death due to any cause); time-to-event analysis of patient survival (univariate).
Supplementary Table 2. Patient survival (defined as death due to any cause); time-to-event analysis of patient survival (univariate).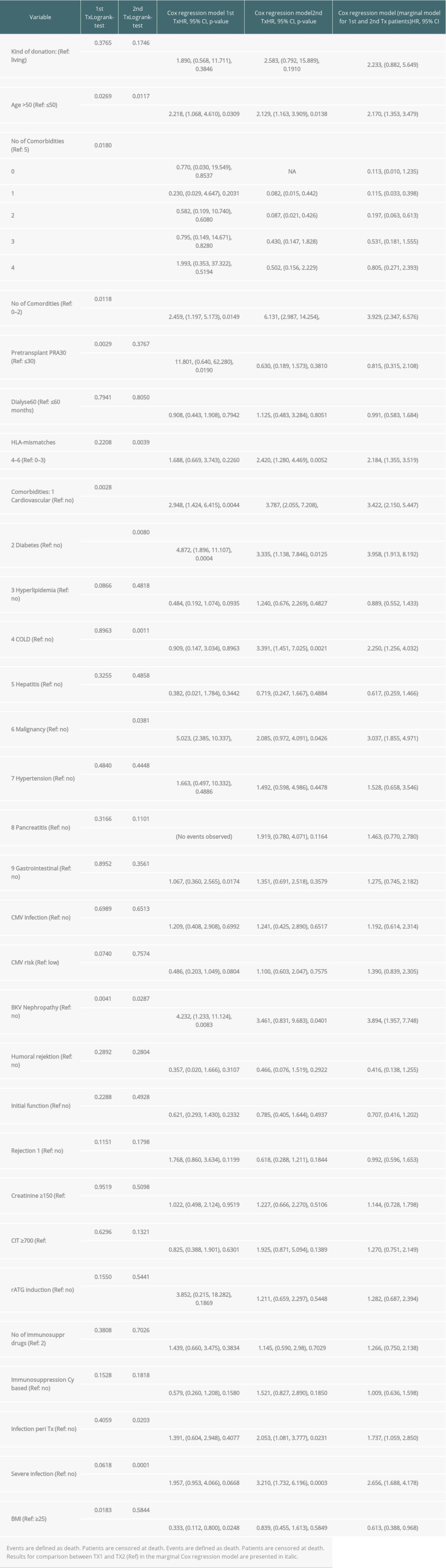
References
1. Husberg BS, Starzl E, The outcome of kidney retransplantation: Arch Surg, 1974; 108; 584-87
2. Haverich A, Haller H, Organtransplantation in Deutschland: Internist, 2016; 57; 7-14 [in German]
3. Schulte K, Borzikowsky C, Rahmel A, Decline in organ donation in Germany – a nationwide secondary analysis of all inpatient cases: Dtsch Arztebl Int, 2018; 115; 463-68
4. Coupel S, Giral-Classe M, Karam G, Ten-year survival of second kidney transplants: Impact of immunologic factors and renal function at 12 months: Kidney Int, 2003; 64; 674-80
5. Magee JC, Barr ML, Basadonna GP, Repeat organ transplantation in the United States, 1996–2005: Am J Transplant, 2007; 7; 1424-33
6. Trébern-Launay K, Foucher Y, Giral M, Poor long-term outcome in second kidney transplantation: A delayed event: PLoS one, 2012; 7; e47915
7. Heaphy ELG, Poggio ED, Flechner SM, Risk factors for retransplant kidney recipients: Relisting and outcomes from patients’ primary transplant: Am J Transplant, 2014; 14; 1356-67
8. Tsapepas DS, Vasilescu R, Tanriover B, Preformed donor-specific antibodies and risk of antibody-mediated rejection in repeat renal transplantation: Transplantation, 2014; 97; 642-47
9. Khalil AK, Slaven J, Mujtaba MA, Re-transplants compared to primary kidney transplants recipients: A mate kidney pared analysis of the OPTN UNOS database: Clin Transplant, 2016; 30; 566-78
10. Clark S, Kadatz M, Gill J, Access to kidney transplantation after a failed first kidney transplant and associations with patient and allograft survival; An analysis of national data to inform allocation policy: Clin J Am Soc Nephrol, 2019; 14; 1228-37
11. Han SH, Go J, Park SC, Long-term outcome of kidney retransplantation in comparison with first transplantation: A propensity score matching analysis: Transplant Proc, 2019; 51; 2582-86
12. Klein JP, Moeschberger ML: Survival analysis, 2003; 235, New York, Berlin, Heidelberg, Springer Verlag
13. Lopez M, Clarkson MR, Albin M, A novel mechanism of action for antithymocyte globulin: Induction of CD4+CD25+Foxp3+ regulatory T cells: J Am Soc Nephrol, 2006; 17; 2844-53
14. Brennan DC, Daller JA, Lake KD, Rabbit antithymoglobulin versus basiliximab in renal transplantation: N Engl J Med, 2006; 355; 1967-77
15. Gurcan S, Luan Y, Dhillon N, Immune reconstitution following rabbit antithymocyte globulin: Am J Transplant, 2010; 10; 2132-41
16. Pankewycz O, Leca N, Kohli R, Low-dose rabbit antithymocyte globulin induction therapy results in prolonged selective lymphocyte depletion irrespective of maintenance immunosuppression: Transplant Proc, 2011; 43; 462-65
17. Issa NC, Fishman JA, Infectious complications of antithymocyte therapies in solid organ transplantation: Clin Infect Dis, 2009; 49; 772-86
18. Lin J, Wang R, Xu Y, Impact of renal allograft nephrectomy on graft and patient survival following retransplantation: A systematic review and meta-analysis: Nephrol Dial Transplant, 2018; 33; 700-8
19. Schachtner T, Otto NM, Stein M, Transplantectomy is associated with presensitization with donor-reactive T cells and graft failure after kidney retransplantation: A cohort study: Nephrol Dial Transplant, 2018; 33; 889-96
20. Badve SV, Paul SK, Klein K, The association between body mass index and mortality in incident dialysis patients: PloS One, 2014; 9; e114897
21. Ku E, Kopple JD, Johansen KL, Longitudinal weight change during CKD progression and its association with subsequent mortality: Am J Kidn Dis, 2018; 71; 657-65
22. Kovesdy CP, Czira ME, Rudas A, Body mass index, waist circumference and mortality in kidney transplant recipients: Am J Transplant, 2010; 10; 2644-51
23. Naik AS, Sakhuja A, Cibrik DM, The impact of obesity on allograft failure after kidney transplantation; A competing risks analysis: Transplantation, 2016; 100; 1963-69
24. Meier-Kriesche HU, Kaplan B, Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: A paired donor kidney analysis: Transplantation, 2002; 74; 1377-81
25. Goldfarb-Rumyantzev A, Hurdle JF, Scandling J, Duration of end-stage renal disease and kidney transplant outcome: Nephrol Dial Transplant, 2005; 20; 167-75
26. Haller MC, Kainz A, Baer H, Dialysis vintage and outcomes after kidney transplantation: A retrospective cohort study: Clin J Am Soc Nephrol, 2017; 12; 122-30
27. Garavoy MR, Rheinschmidt MA, Bogos M, Flow cytometry analysis: A high technology crossmatch technique facilitating transplantation: Transplant Proc, 1983; 15; 1939-40
28. Buelow R, Mercier I, Glanville L, Detection of panel-reactive anti-HLA class I antibodies by enzyme-linked immunosorbent assay or lymphocytotoxicity. Results of a blinded, controlled multicenter study: Hum Immunol, 1995; 44; 1-11
29. Pei R, Lee J-H, Shih N-J, Single human leukocyte antigen flow cytrometric beads for accurate identification of human leucocyte antigen antibody specitificities: Transplantation, 2003; 75; 43-49
30. Lachmann N, Terasaki PI, Budde K, Anti-human leukocyte antigen and donor-specific antibodies detected by Luminex posttransplant serve as biomarkers for chronic rejection of renal allografts: Transplantation, 2009; 87; 1505-13
31. Tait BD, Detection of HLA antibodies in organ transplant recipients – triumphs and challenges of the solid phase bead assays: Front Immunol, 2016; 7; 570-81
32. Lucisano G, Thiruvengadam S, Hassan S, Donor-specific antibodies detected by single antigen beads alone can help risk stratify patients undergoing retransplantation across a repeat HLA mismatch: Am J Transplant, 2020; 20; 441-50
33. Terasaki PI, McCelland JD, Microdroplet assay of human serum cytotoxins: Nature, 1964; 204; 998-1000
34. Patel R, Terasaki PI, Significance of the positive crossmatch test in kidney transplantation: N Engl J Med, 1969; 280; 735-39
35. Helanterä I, Salmela K, Kyllönen L, Pretransplant dialysis duration and risk of death after kidney transplantation in the current era: Transplantation, 2014; 98; 458-64
36. Benkö T, Halfmann P, Gäckler A, Long-term outcome of third, fourth and fifth kidney transplantation: Technical aspects and immunological challenges: Clin Kidney J, 2019; 12; 895-900
37. Fried LP, Tangen CM, Walson J, Frailty in older adults: Evidence for a phenotype: J Gerontol A Biol Sci Med Sci, 2001; 56; M146-56
38. Wilhelm-Leen ER, Hall YN, Tamura MK, Frailty and cfhronic kidney disease: The third national health and nutrition evaluation survey: Am J Med, 2009; 122; 664-71
39. Haugen Ch E, Chu NM, Ying H, Frailty and acces to kidney transplantation: Clin J Am Soc Nephrol, 2019; 14; 576-82
40. McAdams-DeMarco MA, Law A, King E, Frailty and mortality in kidney transplant recipients: Am J Transplant, 2015; 15; 149-54
41. Horvath S, DNA methylation age of human tissues and cell types: Genome Biol, 2013; 14; R115
42. Marioni RE, Sha S, McRae AF, The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936: Int J Epidemiol, 2015; 44; 1388-96
43. Knoll G, Muirhead N, Trpeski L, Patient survival following renal transplant failure in Canada: Am J Transplant, 2005; 5; 1719-24
44. Johnston O, Rose CL, Gill JS, Risks and benefits of preemptive second transplantation: Transplantation, 2013; 95; 705-10
45. Schold JD, Augustine JJ, Huml AM, Modest rates and wide variation in timely access to repeat kidney transplantation in the United States: Am J Transplant, 2020; 20; 769-78
46. Ojo A, Wolfe RA, Agodoa LY, Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: Multivariate analyses from the United States Renal Data System: Transplantation, 1998; 66; 1651-59
47. Rao PS, Schaubel DE, Wei G, Evaluating the survival benefit of kidney retransplantation: Transplantation, 2006; 82; 669-74
Figures
 Figure 1. Cohort TX2 (162 patients after kidney re-transplantation) compared to TX1 (162 matched control patients after first transplantation)Graft survival (p<0.001), as well as patient survival (0.048) were significantly inferior in TX2 compared to TX1 patients.
Figure 1. Cohort TX2 (162 patients after kidney re-transplantation) compared to TX1 (162 matched control patients after first transplantation)Graft survival (p<0.001), as well as patient survival (0.048) were significantly inferior in TX2 compared to TX1 patients. Figure 2. Severe infection endangering graft and/or patient survival(A) Graft and patient survival of 64 TX1 patients with severe infection compared to 98 TX1 patients without were not significantly different. (B) However, graft and patient survival of 67 TX2 patients with severe infection compared to 95 TX2 patients without were significantly inferior (p=0.0332 and p=0.0001, respectively).
Figure 2. Severe infection endangering graft and/or patient survival(A) Graft and patient survival of 64 TX1 patients with severe infection compared to 98 TX1 patients without were not significantly different. (B) However, graft and patient survival of 67 TX2 patients with severe infection compared to 95 TX2 patients without were significantly inferior (p=0.0332 and p=0.0001, respectively). Figure 3. Number of comorbidities(A) Graft survival of 63 TX1 patients with 3–5 compared to 99 TX1 patients with 0–2 comorbidities was not statistically different; while patient survival of TX1 patients with 3–5 comorbidities, was significantly inferior to those with 0–2 (p=0.0118). (B) Graft as well as patient survival of 77 TX2 patients with 3–5 compared to 85 TX2 patients with 0–2 comorbidities were significantly inferior (p=0.001 and p<0.0001 respectively).
Figure 3. Number of comorbidities(A) Graft survival of 63 TX1 patients with 3–5 compared to 99 TX1 patients with 0–2 comorbidities was not statistically different; while patient survival of TX1 patients with 3–5 comorbidities, was significantly inferior to those with 0–2 (p=0.0118). (B) Graft as well as patient survival of 77 TX2 patients with 3–5 compared to 85 TX2 patients with 0–2 comorbidities were significantly inferior (p=0.001 and p<0.0001 respectively). Figure 4. Cardiovascular disease(A) Graft as well as patient survival of 65 TX1 patients with compared to 97 TX1 patients without cardiovascular disease were significantly inferior (p=0.0177 and p=0. 0028, respectively). (B) Graft as well as patient survival in 61 TX2 patients with compared to 101 TX2 patients without cardiovascular disease were significantly inferior. However, in TX2 patients this relationship was more robust than in TX1 patients (p=0.0006 and p=p<0.0001, respectively).
Figure 4. Cardiovascular disease(A) Graft as well as patient survival of 65 TX1 patients with compared to 97 TX1 patients without cardiovascular disease were significantly inferior (p=0.0177 and p=0. 0028, respectively). (B) Graft as well as patient survival in 61 TX2 patients with compared to 101 TX2 patients without cardiovascular disease were significantly inferior. However, in TX2 patients this relationship was more robust than in TX1 patients (p=0.0006 and p=p<0.0001, respectively). Tables
 Table 1. Demographic data of group TX1 (first transplantation) and TX2 (re-transplantation).
Table 1. Demographic data of group TX1 (first transplantation) and TX2 (re-transplantation). Table 2. Outcome of patient group TX2 (re-transplantation) compared to TX1 (first transplantation) serving as control.
Table 2. Outcome of patient group TX2 (re-transplantation) compared to TX1 (first transplantation) serving as control. Table 3A. Time-to-event analysis of graft survival (multivariable model and variable selection).
Table 3A. Time-to-event analysis of graft survival (multivariable model and variable selection). Table 3B. Time-to-event analysis of patient survival (multivariable model and variable selection).
Table 3B. Time-to-event analysis of patient survival (multivariable model and variable selection). Table 4. Severe infections threatening life or graft survival.
Table 4. Severe infections threatening life or graft survival. Table 1. Demographic data of group TX1 (first transplantation) and TX2 (re-transplantation).
Table 1. Demographic data of group TX1 (first transplantation) and TX2 (re-transplantation). Table 2. Outcome of patient group TX2 (re-transplantation) compared to TX1 (first transplantation) serving as control.
Table 2. Outcome of patient group TX2 (re-transplantation) compared to TX1 (first transplantation) serving as control. Table 3A. Time-to-event analysis of graft survival (multivariable model and variable selection).
Table 3A. Time-to-event analysis of graft survival (multivariable model and variable selection). Table 3B. Time-to-event analysis of patient survival (multivariable model and variable selection).
Table 3B. Time-to-event analysis of patient survival (multivariable model and variable selection). Table 4. Severe infections threatening life or graft survival.
Table 4. Severe infections threatening life or graft survival. Supplementary Table 1. Graft survival (defined as time to transplant failure or death); time-to-event analysis of graft survival (univariate).
Supplementary Table 1. Graft survival (defined as time to transplant failure or death); time-to-event analysis of graft survival (univariate). Supplementary Table 2. Patient survival (defined as death due to any cause); time-to-event analysis of patient survival (univariate).
Supplementary Table 2. Patient survival (defined as death due to any cause); time-to-event analysis of patient survival (univariate). In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








