27 November 2020: Original Paper
An Effective Cooling Device for Minimal-Incision Kidney Transplantation
Yansheng Li1ACDEG, Xiuwu Han1ACDEG*, Bayan-Undur Dagvadorj2AFG, Yongwei Zhao34ADF, Xin Zhang1CDG, Xuhui Zhu1BCF, Tao Li1BDF, Peng Zhang1BDF, Yuanhao Chen1BCF, Gao Li1BCF, Lkhamsuren Jambaljav2ADGDOI: 10.12659/AOT.928773
Ann Transplant 2020; 25:e928773
Abstract
BACKGROUND: This study investigated the safety and efficacy of a new cooling device for use in minimal-incision kidney transplantation (MIKT).
MATERIAL AND METHODS: From June 2016 to December 2021, 9 patients underwent MIKT surgery in our hospitals using the new cooling device to maintain hypothermia. We recorded and analyzed information on the etiology, comorbid status, ongoing renal replacement assessment, BMI, HLA mismatching sites of donors and recipients, and perioperative and postoperative clinical data for recipients.
RESULTS: Kidney transplantation was successfully performed in all patients. The kidney surface temperature measurement results showed that the intraoperative renal anterior and posterior surface temperatures were stable at approximately 3.8±1.2°C and 5.2±1.3°C, respectively, during ice-water circulation. The mean operation time was 112±15 min, the artery anastomosis time was 16±6.0 min, and the vein anastomosis time was 14±4.5 min. All recipients recovered uneventfully. The patients were followed up for 6–30 months. Urinary and vascular complications were not found in any recipients.
CONCLUSIONS: The new cooling device can facilitate MIKT. It is safe and feasible to carry out MIKT using the new cooling device, which can reduce surgical trauma and improve the quality of vascular anastomosis with satisfactory cosmetic results.
Keywords: Anastomosis, Surgical, Hypothermia, Induced, Kidney Failure, Chronic, Kidney Transplantation, Surgical Procedures, Minimally Invasive, Cold Temperature, Cryotherapy, Kidney, operative time, Tissue Donors, Vascular Surgical Procedures, young adult
Background
Recipients of renal transplants are patients with kidney failure, often accompanied by anemia, hypoalbuminemia, and edema. The incidence of wound infection, poor wound healing, and other complications after renal transplantation are potentially higher than those in other surgical patients [1]. Therefore, reducing the trauma of kidney transplantation and protecting the donor kidney from all possible adverse factors during the operation are still important clinical goals for transplant surgeons. Surgeons have performed many studies on this topic. The minimal-incision technique for kidney transplantation (MIKT) has been previously reported [2–6]. In recent years, we have carried out a series of animal experiments and clinical studies on laparoscopic kidney transplantation [7–11] and designed a net-restrictive bicirculating double-layer plastic jacket (China, patent number: ZL2018 2 0687164.X). We speculate that it is reasonable and feasible to use this device for MIKT, so we refined this new cooling device and performed the present clinical study to investigate its safety and efficacy in MIKT.
Material and Methods
DESIGN AND ASSEMBLY OF THE NET-RESTRICTIVE PLASTIC JACKET:
The net-restrictive plastic jacket (NPJ) is composed of a homemade sealed plastic bag (approximately 12×20 cm, with 2 silicone or latex tubes) and a small plastic mesh bag. The bottom of the plastic bag is dented to form a new cavity. The donor kidney is put into the cavity, and then the opening of the cavity is closed from both sides with titanium clips, leaving a gap in the middle for the vessels to be placed outside. The plastic bag (including the kidney) is wrapped and trussed tightly with the mesh bag. Then, the mesh bag is cut open (approximately 3 cm) at the renal hilum to expose the renal artery and vein for intraoperative anastomosis. The device was designed to cover almost the entire kidney and is equipped with 2 tubes, with flow formed by the circulating perfusion of saline ice-water at 0–4°C during the operation to achieve a constant and reliable cooling effect (Figure 1).
KIDNEY TRANSPLANT SURGERY:
Before the transplantation, we performed a careful and meticulous back-table preparation of the kidney and completed the assembly of the NPJ, kidney placement, and leakage testing. All components, including temperature-measuring electrode wires, were strictly disinfected. A thread was tied transversely on the surface of the graft kidney between the artery and vein, and the measuring electrode probe was placed and fixed to the thread on both sides of the kidney before the kidney was put into the jacket. Oblique incisions in the right or left hypogastrium were made 3–4 cm above the inguinal ligament and 2–3 cm from the midline at the medial end, with a length of 7–9 cm (Figure 2). During transplantation, the skin, subcutaneous tissue, and external fascia were incised through the minimal incision, and then the internal oblique was carefully separated, and the transverse fascia was incised to create the extraperitoneal space. The abdominal muscularis layer was only cut if necessary, depending on recipient condition. After a sufficient iliac fossa extraperitoneal pouch was formed, the donor kidney and the cooling device were fully placed into the pouch; only the inlet and outlet of the cooling device and the measuring electrode wires crossed the incision (Figure 3).
During the operation, the cooling water was circulated within the cooling system by 2 assistants using two 50-mL injection syringes at a rate of approximately 5 syringes per minute (approximately 250–300 mL/min). One assistant pushed ice-water in the syringe from one tube, and another assistant refilled another syringe with ice-water in a basin that acted as an ice-water reservoir collecting the circulated ice-water from another tube of the NPJ. The renal vein and renal artery were anastomosed end-to-side with the external iliac vein and external iliac artery, respectively, in only 1 position in the pouch. Approximately 3–5 min before the anastomosis was performed (3–5 min before revascularization), we stopped the circulation of the cooling water. Real-time monitoring of graft kidney temperature was performed using a multichannel temperature tester (JK808, Changzhou Jin Ai Lian Electronic Technology Co., Ltd, Changzhou, China) and recorded and shown on a computer screen during renal artery and vein anastomosis (Figure 4).
After completing the renal artery and vein anastomosis, the revascularization of the kidney was performed. Ensuring that there was no active bleeding in the surgical field, the cooling jacket was cut open to free the kidney, all components of the device were removed completely, and the measuring electrode was removed (Figure 5). The ureter and bladder were anastomosed by mucosa-to-mucosa procedures, while a double “J” tube was inserted intraoperatively. Then, the bladder seromuscular layer was intermittently sutured with the 4-0 absorbable suture for 2–3 stitches to bury the anastomosis. After instruments and dressings were accounted for, 1 drainage tube was embedded in the wound, and the incision was closed.
IMMUNOSUPPRESSION:
Patients received intravenous methylprednisolone. The intraoperative dose was 1 g, and the drug was given consecutively for 3 days postoperatively at a dose of 500 mg. The postoperative immunosuppressive protocol of tacrolimus, mycophenolate, and prednisone was used with tacrolimus 2–3 mg twice a day. Oral administration was started on the first day after surgery, and the dosage was adjusted according to the blood-drug concentration. The dosage of mycophenolate was 750 mg, 2 times a day. Oral administration was started on the first day after surgery. Prednisone was administered at 30 mg a day, and oral administration was started 4 days after surgery.
STATISTICAL ANALYSIS:
SPSS 18.0 statistical software (SPSS Inc., Chicago, IL, USA) was used to perform data analysis. Results are expressed as the mean standard deviation (SD).
Results
Kidney transplantation was successfully performed in all patients, and all recipients recovered uneventfully. The kidney surface temperature measurement results showed that the intraoperative renal anterior and posterior surface temperatures were stable at approximately 3.8±1.2°C and 5.2±1.3°C during ice-water circulation (Figures 4, 6). The mean duration of surgery was 112±15 min, the artery anastomosis time was 16±6.0 min, and the vein anastomosis time was 14±4.5 min. The patients were followed up for 6–30 months. Urinary and vascular complications were not found in any recipients. The intraoperative and postoperative observation data of the recipients are shown in Table 3.
Discussion
The potential advantage of reducing incision trauma will benefit those patients with significantly impaired wound healing ability due to immunosuppression. During MIKT, the quality of vascular anastomosis should be ensured while surgical injury should be reduced, including surgical trauma to the recipient and various injuries to the donor kidney. Øyen et al. reported on 21 cases of MIKT and carried out a prospective follow-up control study. The donor kidney was placed in the iliac fossa, the final position of transplantation, to complete the anastomosis of the vessel and the ureter. The results showed that minimal-incision kidney transplantation could be performed safely and rapidly and had beneficial effects on postoperative pain/analgesia, recovery, and complications [2]. Brockschmidt et al. [5] reported on 10 cases of MIKT. They placed the donor kidney outside the body for vascular anastomosis. All recipients had good graft function without complications. Placing the kidney in a cooling bag outside the wound with vessels crossing the abdominal wall through the wound may increase surgical difficulties when performing blood vessel anastomosis with the smaller access. In addition, with the reduced access to perform anastomosis, it may be possible to tear the blood vessels if the tension is not well controlled. Anastomotic quality may be compromised if it is performed under excessive tension. Moreover, during the operation, if the kidney is placed onto the body, the surgeon or assistant must hold it for anastomosis, which may squeeze the graft kidney, accelerate the melting of the ice slush, increase or fluctuate the temperature of the kidney, and waste time when adding ice slush and removing water. Compared with traditional kidney transplantation, the duration of MIKT may be prolonged if the kidney is placed into the body for anastomosis of vessels. Studies have revealed that prolonged anastomosis time leads to significantly inferior long-term graft outcomes and patient survival [12,13]. MIKT may be more likely to become an extensively accepted procedure if the kidney is inserted into the body with reliable hypothermia protection measures for the anastomosis of the renal and iliac vessels. The device used in this study achieved satisfactory results in the clinical application of laparoscopic kidney transplantation [10,11]. In the first laparoscopic kidney transplantation in China, this device worked for 125 min during the surgery, maintaining the temperature at 1–4°C [10].
The advantages of this NPJ are: (1) it can produce a reliable cooling effect and maintain it for the required length of time; (2) the mesh bag can be clamped to adjust the position of the kidney for vascular anastomosis; (3) it can protect the kidney from instrument injury; (4) the mesh bag at the outermost border could limit the overexpansion by wrapping the donor kidney tightly and occupying a small space; and (5) it avoids the disadvantages of the ice gauze jacket that was used traditionally, which needed repeated saline suction and continuous clear visibility. Dependable intraoperative hypothermia can ensure the renal function of transplantation and increase the time limit of vascular anastomosis, which reduces the pressure on the surgeon, thus ensuring the quality of vascular anastomosis. A limitation of this study is the small sample size. The NPJ is made in-house, and it will perform better if it is refined as a commercial product.
Conclusions
The new cooling device NPJ could facilitate MIKT. It is safe and feasible to carry out kidney transplantation with minimal incision using the NPJ to induce hypothermia, which can reduce surgical trauma, protect the kidney from injuries, shorten the duration of vascular anastomosis, improve the quality of vascular anastomosis, achieve satisfactory cosmetic results, and improve graft function. The NPJ should be widely used in clinical transplantation.
Figures
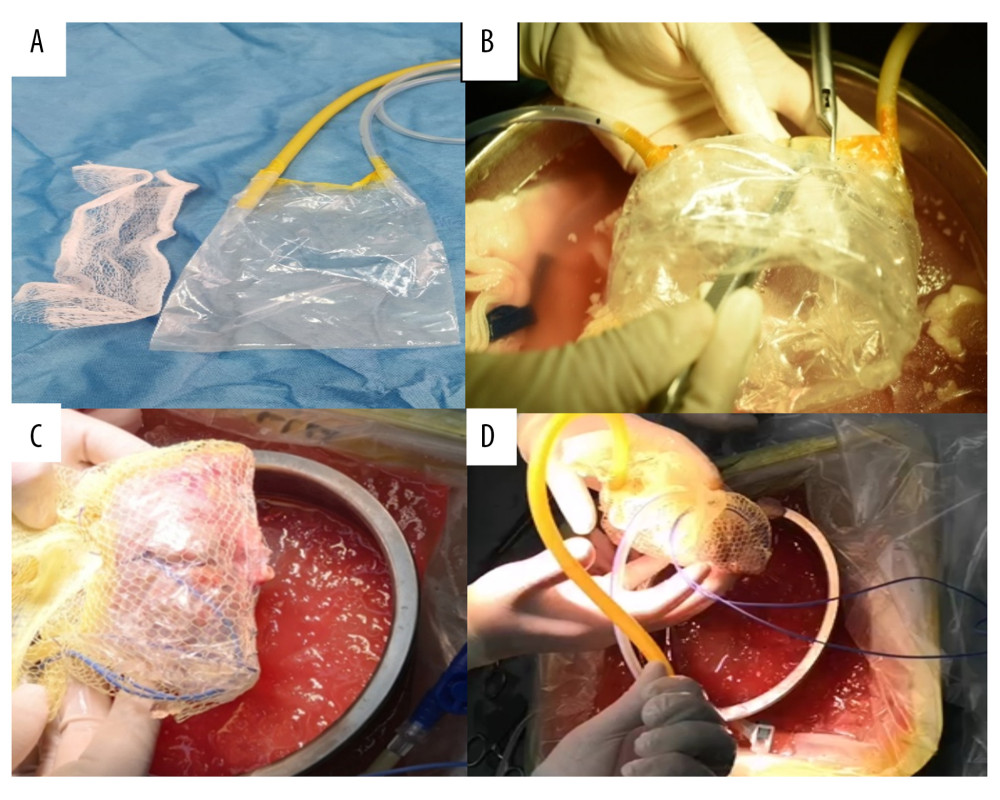 Figure 1. Components and assembly of net-restrictive plastic jacket. (A) Components: plastic bag and plastic net. (B) Denting the bottom of the sealed plastic bag to form a new cavity. (C) Placing the kidney into the cavity and closing the cavity from both sides of the bag with a titanium clip, leaving a gap in the middle for the vessels to be placed outside. Wrapping the plastic bag and kidney with the mesh bag, and opening a hole to place the renal blood vessels outside. (D) Assembly of the net-restrictive plastic jacket finished with tubes and probe.
Figure 1. Components and assembly of net-restrictive plastic jacket. (A) Components: plastic bag and plastic net. (B) Denting the bottom of the sealed plastic bag to form a new cavity. (C) Placing the kidney into the cavity and closing the cavity from both sides of the bag with a titanium clip, leaving a gap in the middle for the vessels to be placed outside. Wrapping the plastic bag and kidney with the mesh bag, and opening a hole to place the renal blood vessels outside. (D) Assembly of the net-restrictive plastic jacket finished with tubes and probe.  Figure 2. Oblique incisions in the right hypogastrium was performed 3–4 cm above the inguinal ligament and 2–3 cm from the midline at the medial end, with a length of 7–9 cm.
Figure 2. Oblique incisions in the right hypogastrium was performed 3–4 cm above the inguinal ligament and 2–3 cm from the midline at the medial end, with a length of 7–9 cm. 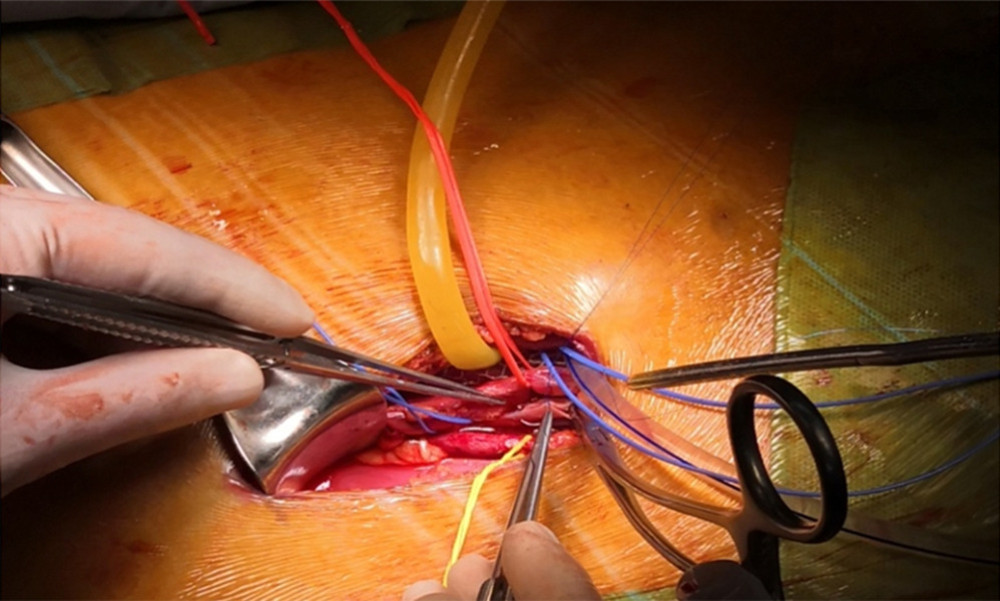 Figure 3. Placement of the donor kidney and the cooling device into the extraperitoneal space for surgeons to complete the renal arteries and veins anastomosis. Only the inlet and outlet of the cooling device and the measuring electrode line crossed the incision.
Figure 3. Placement of the donor kidney and the cooling device into the extraperitoneal space for surgeons to complete the renal arteries and veins anastomosis. Only the inlet and outlet of the cooling device and the measuring electrode line crossed the incision. 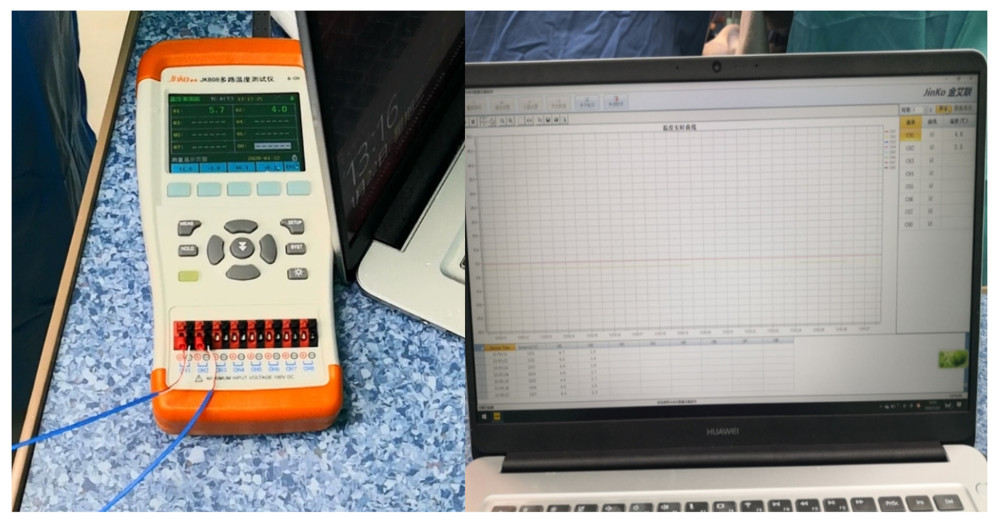 Figure 4. The real-time monitoring of graft kidney temperature during the renal artery and vein anastomosis.
Figure 4. The real-time monitoring of graft kidney temperature during the renal artery and vein anastomosis. 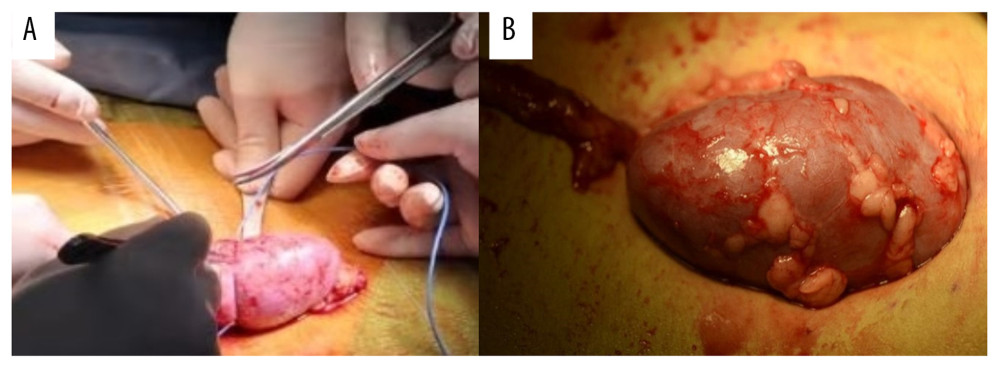 Figure 5. After the completion of revascularization, the transplanted kidney was bright red, with renal artery filling and good pulsation. The cooling jacket was cut to free the kidney, and the surgical team checked and verified that all components were completely removed, and the measuring electrode was removed (A). Minimal incisions can be smaller than the kidney (B).
Figure 5. After the completion of revascularization, the transplanted kidney was bright red, with renal artery filling and good pulsation. The cooling jacket was cut to free the kidney, and the surgical team checked and verified that all components were completely removed, and the measuring electrode was removed (A). Minimal incisions can be smaller than the kidney (B). 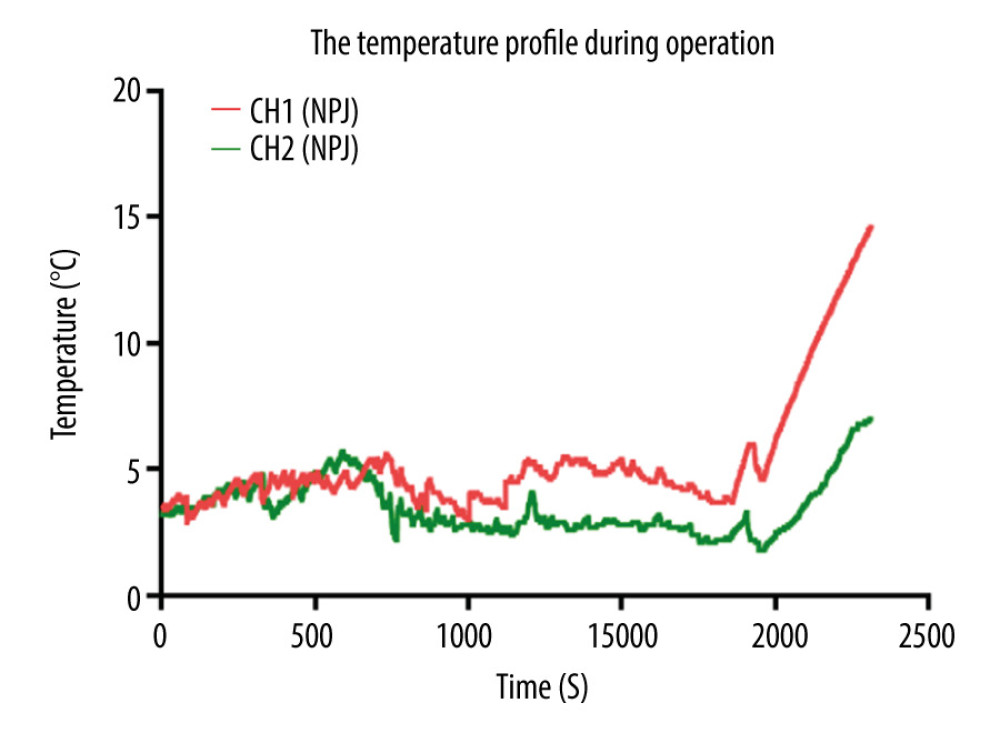 Figure 6. Temperature curves from kidney introduced into cooling devices to kidney revascularization. CH1(NPJ): Net-restrictive plastic jacket cooling surgery, underside. CH2(NPJ): Net-restrictive plastic jacket cooling surgery, upside.
Figure 6. Temperature curves from kidney introduced into cooling devices to kidney revascularization. CH1(NPJ): Net-restrictive plastic jacket cooling surgery, underside. CH2(NPJ): Net-restrictive plastic jacket cooling surgery, upside. References
1. Flechner SM, Zhou L, Derweesh I, The impact of sirolimus, mycophenolate mofetil, cyclosporine, azathioprine, and steroids on wound healing in 513 kidney-transplant recipients: Transplantation, 2003; 76(12); 1729-34
2. Øyen O, Scholz T, Hartmann A, Minimally invasive kidney transplantation: The first experience: Transplant Proc, 2006; 38; 2798-802
3. Kaçar S, Eroğlu A, Tilif S, Minimally Invasive kidney transplantation: Transplant Proc, 2013; 45(3); 926-28
4. Kim SD, Kim JI, Moon IS, Comparison of minimal skin incision technique in living kidney transplantation and conventional kidney transplantation: Chin Med J (Engl), 2016; 129(8); 917-21
5. Brockschmidt C, Huber N, Paschke S, Minimal access kidney transplant: A novel technique to reduce surgical tissue trauma: Exp Clin Transplant, 2012; 10; 319-24
6. Phillips SH, Hill SK, Lipscomb LD, Altering the approach: Open minimally invasive renal transplant in obese patients through the anterior rectus sheath: Urology, 2017; 105; 192-96
7. Han X, Zhang B, Yan W, Comparison of 2 devices in pigs to induce hypothermia in laparoscopic orthotopic kidney transplant: Exp Clin Transplant, 2012; 10(6); 573-78
8. Han X, Zhao Y, He B, Feasibility of laparoscopic combined para-orthotopic pancreas and orthotopic kidney transplantation: Initial research with a pig model: Ann Transplant, 2018; 23; 879-87
9. Han X, Zhao Y, Zhu X, Vein “Beltization” technique facilitates venous anastomoses for laparoscopic orthotopic kidney transplantation in a pig model: Transplant Proc, 2019; 51(3); 913-18
10. Han X, Zhao Y, Zhang XLaparoscopic kidney transplantation: 1 case report: Shandong Medicine, 2018; 58(46); 63-65 [in Chinees]
11. Li S, Han X, Li TNursing experience of 2 cases of central renal carcinoma treated by laparoscopic autologous kidney transplantation: General Nursing, 2019; 17(36); 4557-59 [in Chinees]
12. Marzouk K, Lawen J, Alwayn I, The impact of vascular anastomosis time on early kidney transplant outcomes: Transplant Res, 2013; 2; 8
13. Weissenbacher A, Oberhuber R, Cardini B, The faster the better: Anastomosis time influences patient survival after deceased donor kidney transplantation: Transpl Int, 2015; 28; 535-43
Figures
 Figure 1. Components and assembly of net-restrictive plastic jacket. (A) Components: plastic bag and plastic net. (B) Denting the bottom of the sealed plastic bag to form a new cavity. (C) Placing the kidney into the cavity and closing the cavity from both sides of the bag with a titanium clip, leaving a gap in the middle for the vessels to be placed outside. Wrapping the plastic bag and kidney with the mesh bag, and opening a hole to place the renal blood vessels outside. (D) Assembly of the net-restrictive plastic jacket finished with tubes and probe.
Figure 1. Components and assembly of net-restrictive plastic jacket. (A) Components: plastic bag and plastic net. (B) Denting the bottom of the sealed plastic bag to form a new cavity. (C) Placing the kidney into the cavity and closing the cavity from both sides of the bag with a titanium clip, leaving a gap in the middle for the vessels to be placed outside. Wrapping the plastic bag and kidney with the mesh bag, and opening a hole to place the renal blood vessels outside. (D) Assembly of the net-restrictive plastic jacket finished with tubes and probe. Figure 2. Oblique incisions in the right hypogastrium was performed 3–4 cm above the inguinal ligament and 2–3 cm from the midline at the medial end, with a length of 7–9 cm.
Figure 2. Oblique incisions in the right hypogastrium was performed 3–4 cm above the inguinal ligament and 2–3 cm from the midline at the medial end, with a length of 7–9 cm. Figure 3. Placement of the donor kidney and the cooling device into the extraperitoneal space for surgeons to complete the renal arteries and veins anastomosis. Only the inlet and outlet of the cooling device and the measuring electrode line crossed the incision.
Figure 3. Placement of the donor kidney and the cooling device into the extraperitoneal space for surgeons to complete the renal arteries and veins anastomosis. Only the inlet and outlet of the cooling device and the measuring electrode line crossed the incision. Figure 4. The real-time monitoring of graft kidney temperature during the renal artery and vein anastomosis.
Figure 4. The real-time monitoring of graft kidney temperature during the renal artery and vein anastomosis. Figure 5. After the completion of revascularization, the transplanted kidney was bright red, with renal artery filling and good pulsation. The cooling jacket was cut to free the kidney, and the surgical team checked and verified that all components were completely removed, and the measuring electrode was removed (A). Minimal incisions can be smaller than the kidney (B).
Figure 5. After the completion of revascularization, the transplanted kidney was bright red, with renal artery filling and good pulsation. The cooling jacket was cut to free the kidney, and the surgical team checked and verified that all components were completely removed, and the measuring electrode was removed (A). Minimal incisions can be smaller than the kidney (B). Figure 6. Temperature curves from kidney introduced into cooling devices to kidney revascularization. CH1(NPJ): Net-restrictive plastic jacket cooling surgery, underside. CH2(NPJ): Net-restrictive plastic jacket cooling surgery, upside.
Figure 6. Temperature curves from kidney introduced into cooling devices to kidney revascularization. CH1(NPJ): Net-restrictive plastic jacket cooling surgery, underside. CH2(NPJ): Net-restrictive plastic jacket cooling surgery, upside. Tables
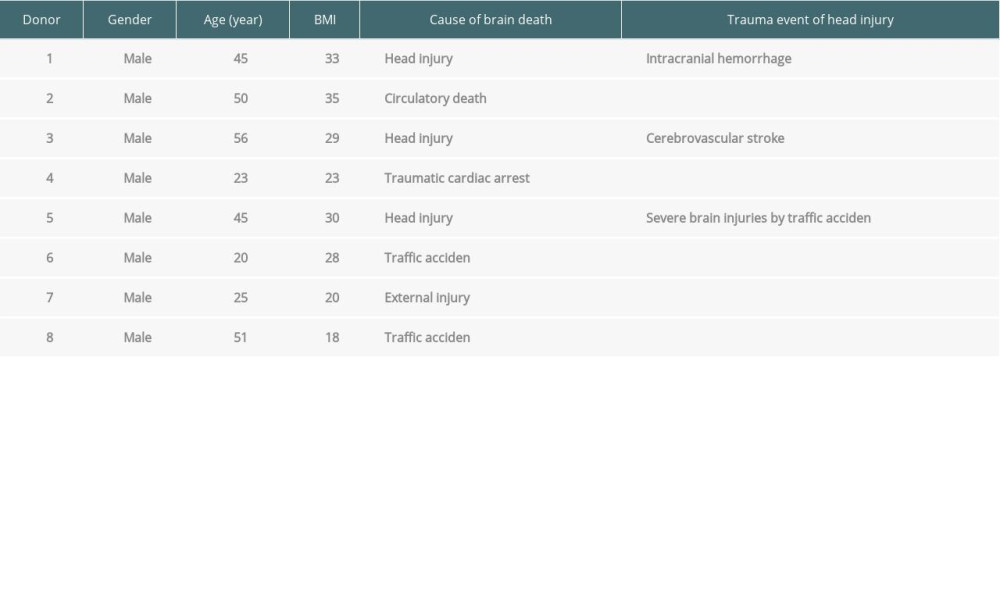 Table 1. The medical reasons for brain death of the donors.
Table 1. The medical reasons for brain death of the donors.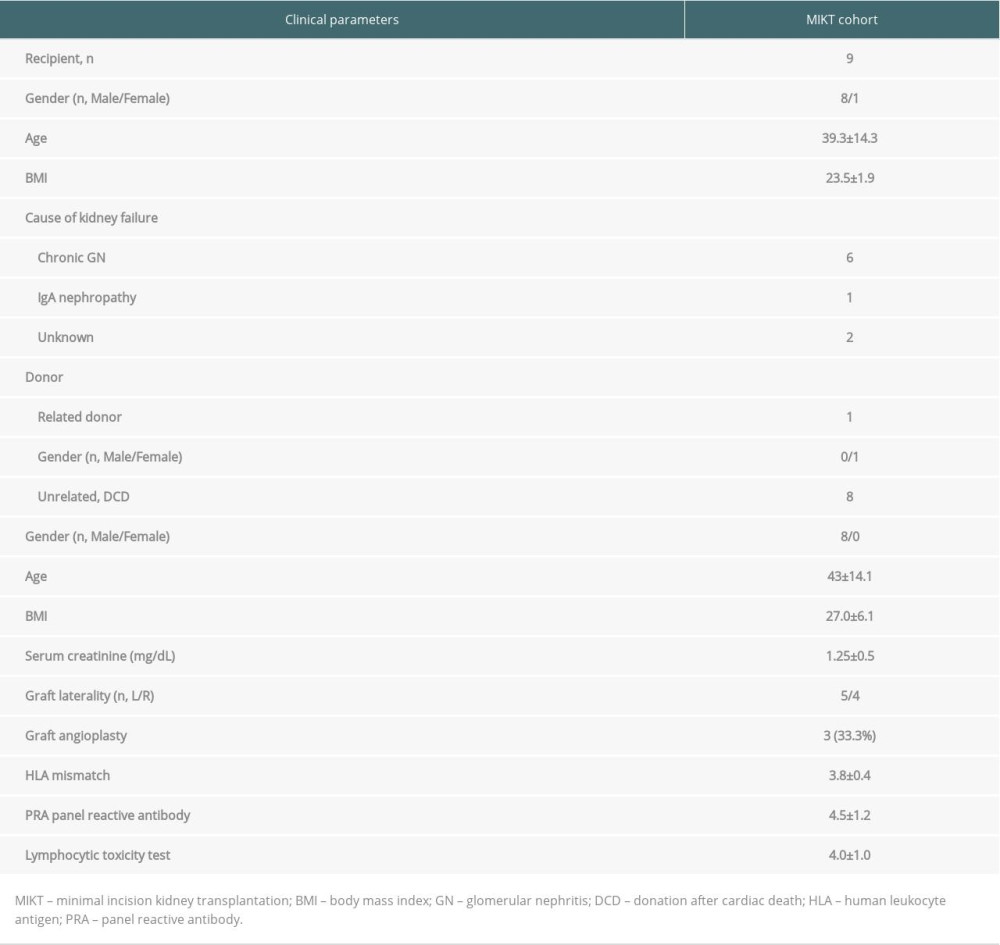 Table 2. Summary of characteristics of donor and recipient.
Table 2. Summary of characteristics of donor and recipient.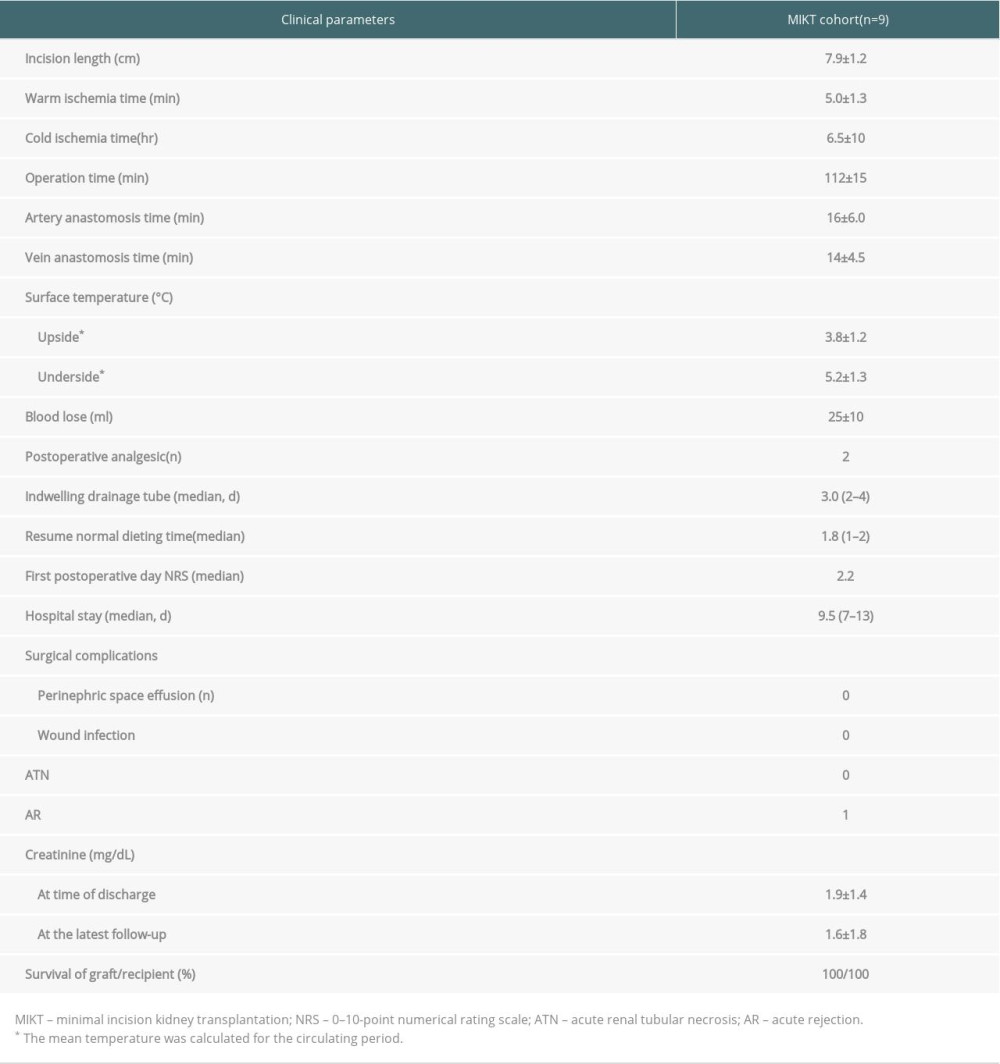 Table 3. Perioperative and postoperative clinical data for recipients.
Table 3. Perioperative and postoperative clinical data for recipients. Table 1. The medical reasons for brain death of the donors.
Table 1. The medical reasons for brain death of the donors. Table 2. Summary of characteristics of donor and recipient.
Table 2. Summary of characteristics of donor and recipient. Table 3. Perioperative and postoperative clinical data for recipients.
Table 3. Perioperative and postoperative clinical data for recipients. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








