05 February 2021: Original Paper
Analysis of Risk Factors Affecting Incidence of Osteoporosis and Fragility Fractures in Liver Transplant Recipients
Senichiro Yanagawa1BCDEF, Hiroyuki Tahara1ADEFG*, Yuka Tanaka1B, Seiichi Shimizu1B, Masahiro Ohira1B, Kentaro Ide1B, Hideki Ohdan1DFGDOI: 10.12659/AOT.925475
Ann Transplant 2021; 26:e925475
Abstract
BACKGROUND: Fragility fractures caused by osteoporosis are common complications seen in recipients of organ transplantation who survive long term. Although many risk factors have been identified for osteoporosis after organ transplantation, none of them have been recognized as the main cause of development of the condition. Several studies have examined vitamin D receptor (VDR) gene single-nucleotide polymorphisms (SNPs) for their influence on bone mineral density (BMD) and fracture risk, but with variable results. We aimed to elucidate the risk factors that affect incidence of osteoporosis and fragility fractures in liver transplant recipients.
MATERIAL AND METHODS: In this study, we monitored incidence of fragility fracture and osteoporosis in 45 patients who had been evaluated with dual-energy X-ray absorptiometry (DXA) after liver transplantation. We also analyzed the association between VDR SNPs such as BsmI, ApaI, FokI, and TaqI with osteoporosis and fracture incidence in 27 patients in our cohort in whom SNPs were evaluated and DXA performed after liver transplantation.
RESULTS: Osteoporosis was diagnosed in 17 of 45 patients in whom BMD was measured after liver transplantation. Of the patients with osteoporosis, 15 (88.2%) subsequently had fragility fractures. The incidence of postoperative osteoporosis was significantly higher in the recipients who had alcoholic liver cirrhosis as their primary disease. Interestingly, there were significantly more patients with a homozygous BsmI GG genotype in the group diagnosed with osteoporosis.
CONCLUSIONS: Our study suggests that patients who undergo liver transplantation and have alcoholic liver cirrhosis or the BsmI GG genotype may be at increased risk for osteoporosis. Further research is necessary to confirm these findings.
Keywords: Liver Transplantation, Osteoporosis, Polymorphism, Single Nucleotide, Receptors, Calcitriol, Bone Density, Carcinoma, Hepatocellular, Fractures, Bone, Incidence, Liver Neoplasms, Risk Factors
Background
Fragility fractures caused by osteoporosis are common complications seen in long-term survivors of organ transplantation. These fractures lead to a decrease in activities of daily living and often affect the prognosis of a transplant recipient [1]. A rapid decrease in bone mineral density (BMD) frequently occurs soon after transplantation and is accompanied by a marked increase in fracture probability. Various factors, including long-term use of steroids, aging, undernutrition, and hypogonadism, can predispose an individual to osteoporosis [2,3]. Although many risk factors for osteoporosis after organ transplantation have been identified, a single causative factor has not been identified in development of the condition.
In patients with end-stage cirrhosis who are awaiting liver transplantation, bone metabolism abnormalities are usually advanced. Liver dysfunction, which plays an important role in bone metabolism, promotes osteoporosis after liver transplantation. Therefore, it would be very useful if development of postoperative osteoporosis could be predicted before liver transplantation in patients with end-stage cirrhosis. It has been reported that BMD before transplantation does not predict fracture incidence after liver transplantation [4]. Studies of candidate genes and genome-wide association have identified single-nucleotide polymorphisms (SNPs) associated with osteoporosis incidence. SNPs in several genes have been shown to affect BMD [5]. Among the candidate genes, the vitamin D receptor gene (
Material and Methods
STUDY PATIENTS:
The cohort for the present study consisted of 253 consecutive patients who underwent transplantation with a primary living or deceased donor liver at the Hiroshima University Hospital from June 1991 to December 2017. Of these patients, 45 recipients who had been randomly evaluated with dual-energy X-ray absorptiometry (DXA; Hologic QDR-1000) after their liver transplant were enrolled. In all cases, more than 1 year had passed since the liver transplantation. In detail, BMD measurement was performed in 21 patients within 1 to 2 years after transplantation; in 9, it was done 2 to 3 years later; and in 15 patients, BMD was measured 3 years later. In patients with and without osteoporosis or fragility fractures, age, sex, percentage of postmenopausal women, serum levels of bone metabolism markers before transplantation (Ca, phosphorus [P], intact parathyroid hormone [PTH]), body mass index (BMI) at the time of DXA, duration of steroid therapy after transplantation, percentage of individuals with cirrhosis due to hepatitis C virus (HCV), long-term use of diuretics (>1 month) after transplantation, and presence or absence of Type 2 diabetes mellitus (T2DM) were compared. Using peripheral blood-derived DNA collected before liver transplantation, we analyzed
DNA EXTRACTION AND VDR GENOTYPING:
Genomic DNA was extracted from the recipients’ peripheral blood mononuclear cells using the Wizard SV Genomic DNA Purification System (Promega Corporation, Madison, Wisconsin, United States) according to the manufacturer’s protocol. DNA quality was determined by 1% agarose gel electrophoresis followed by staining with ethidium bromide. Purity of DNA was determined by measuring the optical density of the samples at 260 nm and 280 nm using a Nanodrop Analyzer spectrophotometer. Polymerase chain reaction (PCR)-restriction fragment length polymorphism analysis was used to identify the VDR polymorphisms FokI (T/Crs2228570), BsmI (G/A rs1544410), ApaI (G/T rs7975232), and TaqI (T/C rs731236), as previously described [12]. The primers and restriction enzymes used in this study are listed in Table 1. PCR was performed as follows: An initial denaturation cycle at 95°C for 3 minutes was followed by annealing for 30 cycles at 95°C for 30 seconds, incubation at primer-specific temperatures for 30 seconds and 72°C for 30 seconds, and the final 1-step extension at 72°C for 5 minutes. The restriction endonucleases FokI, BsmI, ApaI, and TaqI were used to digest polymorphic sites of the VDR gene. Enzyme-specific restriction reactions were performed for 5 minutes at 37°C for the BsmI, FokI, and ApaI enzymes and at 65°C for the TaqI enzyme.
DIAGNOSTIC CRITERIA FOR OSTEOPOROSIS AND FRAGILITY FRACTURE:
Osteoporosis was diagnosed by calculating the T score and measuring the YAM of BMD in the lumbar vertebrae of patients using DXA. Following the Japan Osteoporosis Society guidelines for the prevention and treatment of osteoporosis (2015 edition) [13], osteoporosis was defined in this study if a patient met 1 of the following 3 criteria: T score ≤−2.5 standard deviations (SD), YAM <70%, or YAM <80% with bone fracture. A fragility fracture was defined as a non-traumatic fracture caused by a slight external force, with the target fracture sites including the thoracic spine, proximal femur, rib, pelvis, proximal humerus, distal rib, and lower femur.
STATISTICAL ANALYSES:
Data were reported as means±SD or means±standard error of mean for continuous variables and as frequencies (%) for categorical variables. The incidence of fragility fractures, diagnostic markers for BMD (T score and YAM), and clinical risk factors were compared in individuals with and without osteoporosis, using the
Results
RELATIONSHIP BETWEEN OSTEOPOROSIS AND FRAGILITY FRACTURE INCIDENCE IN LIVER TRANSPLANT RECIPIENTS:
Table 2 lists the clinical characteristics of 45 participants. The average age was 56.4 years, with 23 men and 22 women. There was no bias in the primary diseases. Table 3 shows the relationship between diagnostic indicators of BMD (T score and YAM) and fragility fracture incidence. In both posterior-anterior and lateral views of the lumbar vertebrae, all of the indicators were significantly correlated with fracture incidence (P<0.01). Fragility fractures were observed in 32 (12.6%) of the 253 liver transplant recipients in our hospital. Of the 45 patients who underwent DXA scan after liver transplantation, 17 were diagnosed with osteoporosis. Of them, 15 patients (88.2%) subsequently developed fragility fractures. In contrast, only 4 of 28 patients (14.3%) who did not meet the criteria for osteoporosis had fragility fractures, indicating that a diagnosis of osteoporosis positively correlated with subsequent fragility fracture incidence in liver transplant recipients (Table 4).
CLINICAL RISK FACTORS FOR OSTEOPOROSIS:
We assessed the effects of several common clinical risk factors [14,15] on osteoporosis incidence in the 45 recipients examined with DXA after liver transplantation. There were no significant differences in age, percentage of postmenopausal women, bone metabolism markers (Ca, P, PTH), BMI, steroid exposure period, percentage of individuals with cirrhosis due to HCV, long-term use of diuretics, or T2DM between the patients with and without osteoporosis. However, incidence of postoperative osteoporosis was significantly higher in the transplant recipients who had alcoholic liver cirrhosis as their primary disease than in the other patients (23.5% vs 3.8%, P=0.039) (Table 4).
CLINICAL RISK FACTORS FOR FRAGILITY FRACTURES:
We also assessed the effects of several common clinical risk factors on fragility fractures in the 45 recipients examined with DXA after liver transplantation. However, there were no significant differences in age, percentage of postmenopausal women, serum bone metabolism markers (Ca, P, PTH), BMI, steroid exposure period, percentage of individuals with cirrhosis due to HCV, long-term use of diuretics, or T2DM between the patients with and without osteoporosis (Table 5).
RELATIONSHIP BETWEEN VDR SNPS AND OSTEOPOROSIS:
In 27 of 45 patients who underwent DXA after liver transplantation, 4 VDR SNPs (FokI, BsmI, ApaI, and TaqI) were studied. Seventeen of the 27 patients were diagnosed with osteoporosis. There were no significant allele differences in FokI, ApaI, or TaqI polymorphisms between the patients with and without osteoporosis (Figure 1). However, significantly more patients diagnosed with osteoporosis had the homozygous BsmI GG genotype, whereas no significant differences in BsmI alleles were observed in the non-osteoporosis group, indicating that liver transplant candidates with the BsmI GG genotype had an increased risk of osteoporosis (P=0.021, Figure 1).
RELATIONSHIP BETWEEN VDR SNPS AND FRAGILITY FRACTURE INCIDENCE:
The relationship between each VDR SNP and fragility fracture incidence is shown in Figure 2. In the 27 patients who underwent DXA after liver transplantation and who had fragility fractures after liver transplantation, each VDR SNP was analyzed. There were no significant differences in SNP polymorphism genotypes between patients with and without fragility fractures. In particular, the BsmI GG genotype, which was significantly associated with osteoporosis, did not correlate with fragility fracture incidence.
Discussion
We first analyzed the relationship between osteoporosis and fragility fracture incidence in liver transplant recipients. The diagnosis of osteoporosis in the present study was positively correlated with subsequent fragility fracture incidence. We also found that having alcoholic liver cirrhosis as the primary disease was a clinical risk factor for osteoporosis after liver transplantation. Furthermore,
Recent medical advances have remarkably improved the survival rate of and prognosis for patients after organ transplantation. It is now necessary to improve management strategies for these individuals to reduce their risk of long-term complications. One such complication is the occurrence of fragility fractures after liver transplantation, which impair activities of daily life and thus may affect the prognosis for recipients who have overcome hepatic failure. Osteoporosis, defined as decreased BMD, is closely correlated with deterioration of bone tissue and disruption of bone microarchitecture; therefore, it could increase risk of fragility fractures [16]. Our data also showed a significant close relationship between osteoporosis and liver transplantation. In patients who have undergone liver transplantation, osteoporotic changes progress rapidly, due to undernutrition, loss of exercise, and/or decrease in physical strength, especially in the first year after surgery [17,18]. The recipients, therefore, remain at risk of subsequent fractures in the earlier postoperative period. Furthermore, similar osteoporotic changes also are seen in many patients with cirrhosis who are awaiting liver transplantation. Several reports have shown, however, that preoperative BMD values in candidates for liver transplantation do not correlate with incidence of postoperative osteoporosis or fragility fracture [19,20]. Rapid postoperative bone mineral loss is an important risk factor for subsequent fragility fracture. Administration soon after liver transplantation of various drugs for osteoporosis, such as risedronate, denosumab, and ibandronate, reportedly is effective in suppressing the decrease in BMD [21–23].
Many studies have shown that pre-transplant bone disease and post-transplant exposure to immunosuppressants, particularly high-dose glucocorticoid therapy, are 2 major risk factors for osteoporosis after organ transplantation [24]. Glucocorticoids likely affect bone and mineral homeostasis indirectly, decreasing calcium absorption from the intestine and increasing renal excretion of calcium. Immunosuppressive drugs could also affect bone remodeling. Lan et al hypothesized about possible pathogenetic mechanisms of post-transplant osteoporosis and fractures caused by immunosuppressive agents such as glucocorticoids, calcineurin inhibitors, and mammalian target of rapamycin inhibitors [24]. Holm et al reported on a dynamic time trend in primary osteoporosis risk factors over 12 years since 2000. The prevalent risk factors included age, BMI, T2DM, major osteoporotic fracture history, calcium supplementation, and use of thiazide and high-dose prednisone [25]. In addition, many other studies have reported that primary or secondary osteoporosis can be triggered by smoking, alcohol overdose, estrogen deficiency, menopause, malnutrition, use of immunosuppressant therapy, neurodegenerative disease, secondary parathyroid hyperactivity, liver cirrhosis, and hematological disease. In our retrospective study, age, menopause, BMI, long-term glucocorticoid administration, T2DM, cirrhosis due to HCV, bone metabolism markers including PTH level, and long-term use of diuretics were not identified as risk factors. However, alcoholic liver cirrhosis was identified as a risk factor for osteoporosis. Heavy alcohol consumption has been associated with low BMD. González-Reimers et al demonstrated that alcoholism can cause decreased bone synthesis and/or increased bone breakdown [26]. In prospective studies, the risk of bone loss and fracture after liver transplantation was related to BMD and bone turnover was low in many patients with liver failure. Patients with cirrhosis had a higher prevalence of vitamin D deficiency, and the levels were significantly lower in patients with a class C Child-Pugh score.
Vitamin D is an important factor for BMD metabolism. It potentiates intestinal absorption of Ca, PTH secretion, and bone turnover. These actions are mediated through
The present study was a retrospective analysis of BMD and
A prospective study with a large number of patients should be performed in the future to accurately identify risk factors for osteoporosis and fragility fracture after liver transplantation.
Conclusions
In conclusion, BMD evaluation using DXA after transplantation may prove useful as a diagnostic tool that could predict risk of subsequent fragility fracture. Our analysis of clinical factors and relevant SNPs showed that postoperative osteoporosis in liver transplant recipients was more common in patients with alcoholic liver cirrhosis and in individuals with the
Figures
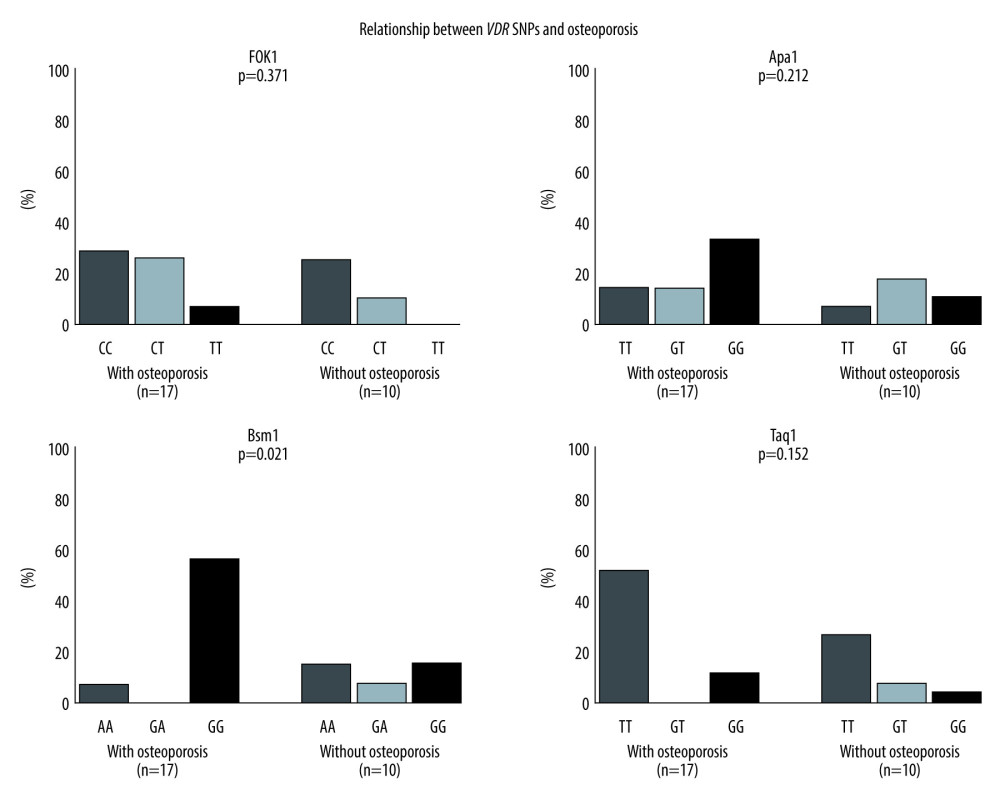 Figure 1. Both bone mineral density and single-nucleotide polymorphisms were measured in 27 patients. For Fok1, Apa1, and Taq1, there were no differences related to osteoporosis. However, there was a significant difference in incidence of osteoporosis associated with the VDR (Bsm1) GG group. * P=0.021.
Figure 1. Both bone mineral density and single-nucleotide polymorphisms were measured in 27 patients. For Fok1, Apa1, and Taq1, there were no differences related to osteoporosis. However, there was a significant difference in incidence of osteoporosis associated with the VDR (Bsm1) GG group. * P=0.021. 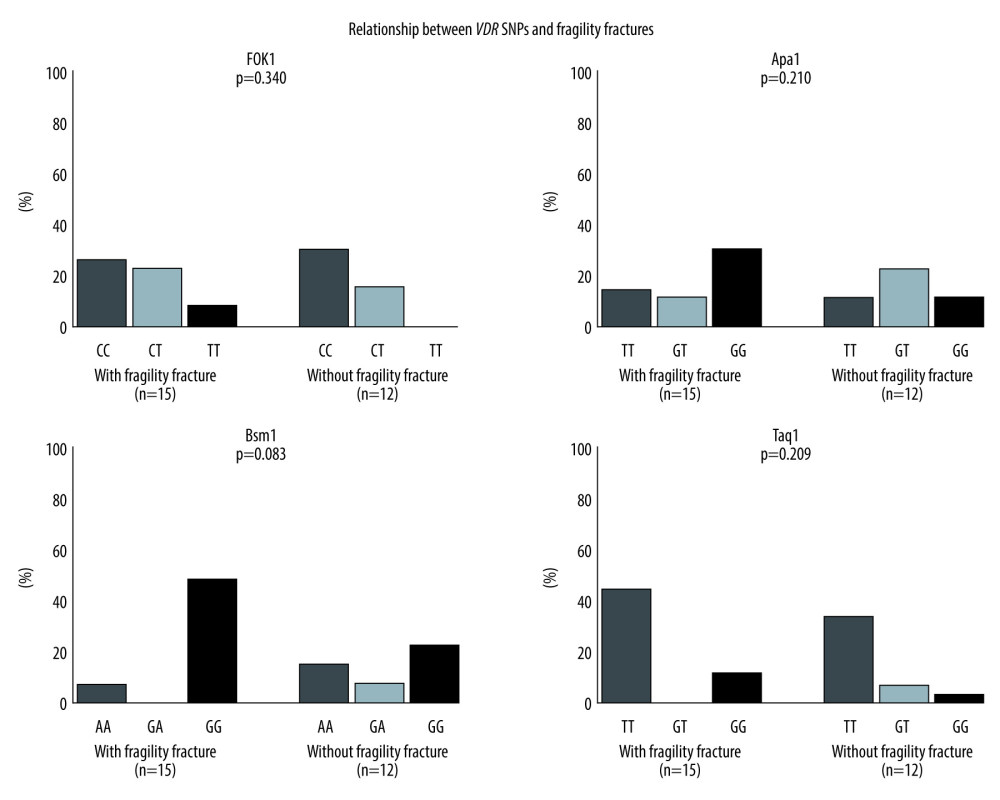 Figure 2. Both bone mineral density and single-nucleotide polymorphisms (SNPs) were measured in 27 patients. There were no differences related to fragility fracture for any of the SNPs.
Figure 2. Both bone mineral density and single-nucleotide polymorphisms (SNPs) were measured in 27 patients. There were no differences related to fragility fracture for any of the SNPs. Tables
Table 1. Primers and restriction enzymes used in the study. Table 2. Characteristics of the patients in whom bone mineral density was assessed (n=45).
Table 2. Characteristics of the patients in whom bone mineral density was assessed (n=45).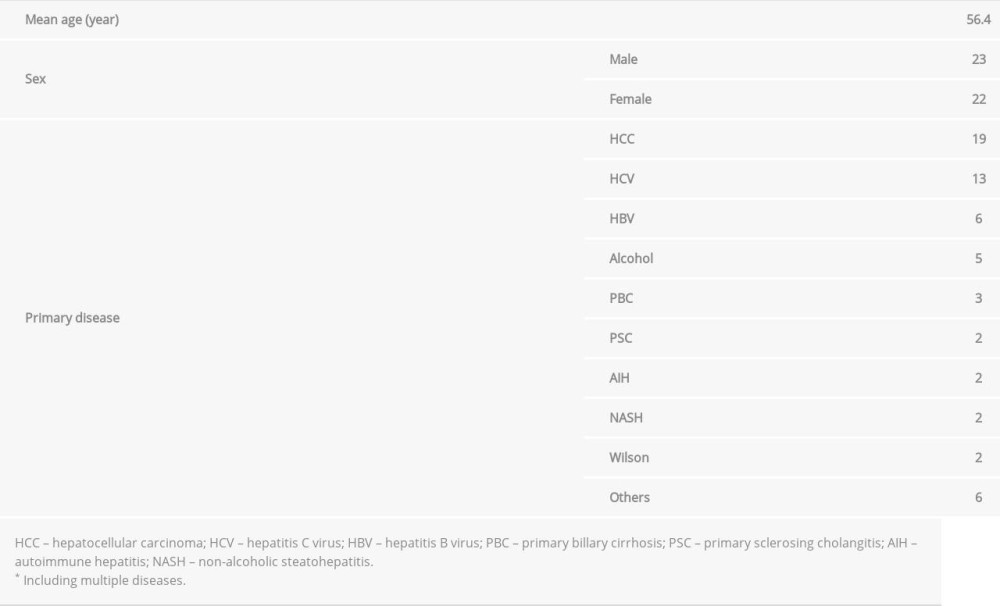 Table 3. Relationship between bone mineral density and fragility fracture. There was a significant difference in frontal or lateral T-scores and young adult mean between the patients with and without fragility fractures.
Table 3. Relationship between bone mineral density and fragility fracture. There was a significant difference in frontal or lateral T-scores and young adult mean between the patients with and without fragility fractures.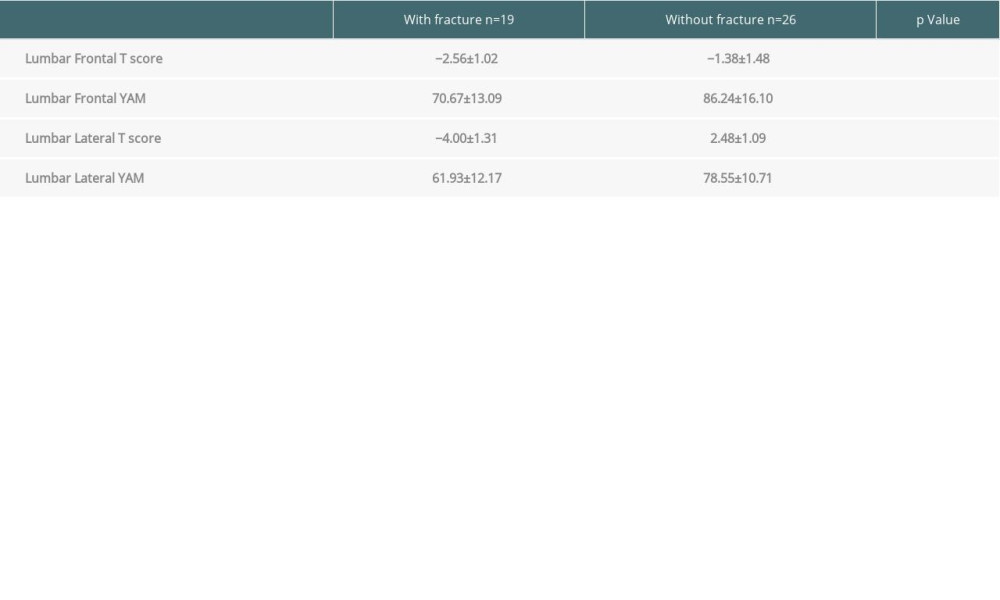 Table 4. There were no significant differences between the groups with and without osteoporosis in bone metabolism marker abnormalities, sex, presence or absence of menopause, body mass index, hepatitis C virus-related disease, or duration of administration of steroids or diuretics. A difference was found in the incidence of fragility fracture due to osteoporosis only in the patients with alcoholic liver cirrhosis.
Table 4. There were no significant differences between the groups with and without osteoporosis in bone metabolism marker abnormalities, sex, presence or absence of menopause, body mass index, hepatitis C virus-related disease, or duration of administration of steroids or diuretics. A difference was found in the incidence of fragility fracture due to osteoporosis only in the patients with alcoholic liver cirrhosis.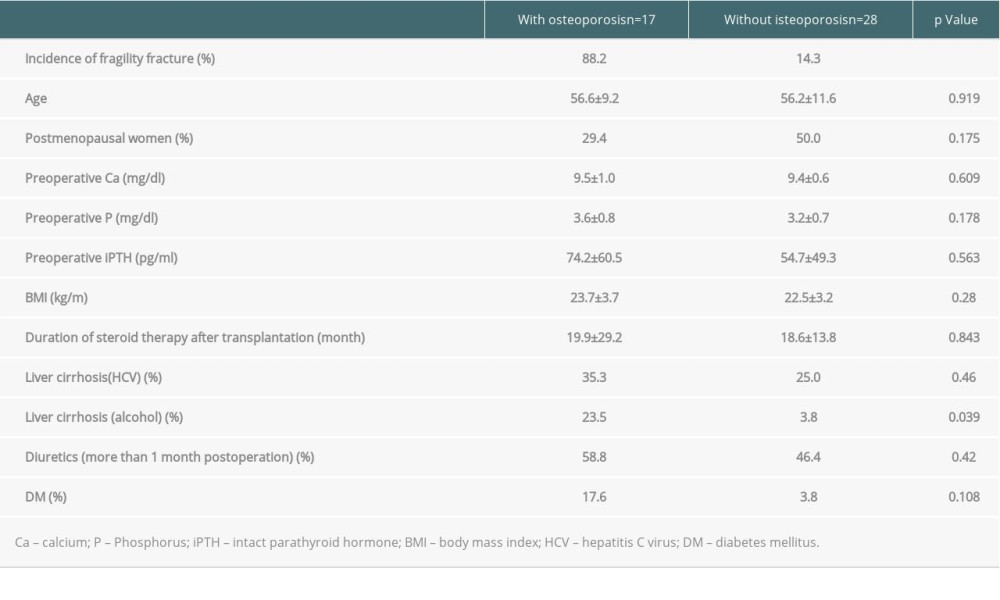 Table 5. There were no significant differences between the groups with and without fragility fractures in bone metabolism marker abnormalities, sex, presence or absence of menopause, body mass index, hepatitis C virus-related disease, alcohol-related disease, or duration of administration of steroids or diuretics.
Table 5. There were no significant differences between the groups with and without fragility fractures in bone metabolism marker abnormalities, sex, presence or absence of menopause, body mass index, hepatitis C virus-related disease, alcohol-related disease, or duration of administration of steroids or diuretics.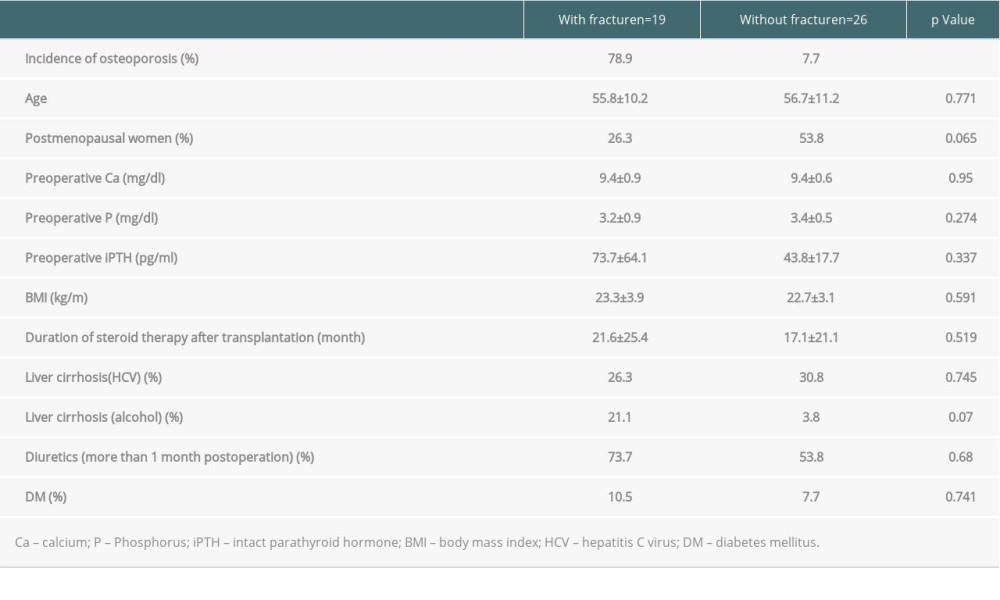
References
1. Ramsey-Goldman R, Dunn JE, Dunlop DD, Increased risk of fracture in patients receiving solid organ transplants: J Bone Miner Res, 1999; 14; 456-63
2. Lan GB, Xie XB, Peng LK, Pathogenesis and management: Biomed Res Int, 2015; 2015; 413169
3. Kulak CA, Borba VZ, Kulak J, Osteoporosis after transplantation: Curr Osteoporos Rep, 2012; 10; 48-55
4. Krol CG, Dekkers OM, Kroon HM, No association between BMD and prevalent vertebral fractures in liver transplant recipients at time of screening before transplantation: J Clin Endocrinol Metab, 2014; 99; 3677-85
5. Nelson DA, Kleerekoper M, The search for the osteoporosis gene: J Clin Endocrinol Metab, 1997; 82; 989-90
6. Haussler MR, Whitfield GK, Haussler CA, Haussler CA, The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed: J Bone Miner Res, 1998; 13; 325-49
7. Gennari L, Merlotti D, De Paola V, Update on the pharmacogenetics of the vitamin D receptor and osteoporosis: Pharmacogenomics, 2009; 10; 417-33
8. Morrison NA, Qi JC, Tokita A, Prediction of bone density from vitamin D receptor alleles: Nature, 1994; 367; 284-87
9. Sainz J, Van Tornout JM, Loro ML, Vitamin D-receptor gene polymorphisms and bone density in prepubertal American girls of Mexican descent: N Engl J Med, 1997; 337; 77-82
10. Zhang L, Yin X, Wang J, Associations between VDR gene polymorphisms and osteoporosis risk and bone mineral density in postmenopausal women: A systematic review and meta-analysis: Sci Rep, 2018; 8; 981
11. Urano T, Inoue S, Genetics of osteoporosis: Biochem Biophys Res Commun, 2014; 452; 287-93
12. Kılıç S, Sılan F, Hız MM, Vitamin D receptor gene BSMI, FOKI, APAI, and TAQI polymorphisms and the risk of atopic dermatitis: J Investig Allergol Clin Immunol, 2016; 26; 106-10
13. The Committee for Prevention and Treatment of Osteoporosis (JAPAN): 2015 Guidelines for Prevention and Treatment of Osteoporosis, 2015, Tokyo, Japan Osteoporosis Society
14. Sözen T, Özışık L, Başaran NÇ, An overview and management of osteoporosis: Eur J Rheumatol, 2017; 4; 46-56
15. Yoshimura N, Oka H, Risk factors for osteoporosis in Japan: Clin Calcium, 2005; 15; 1457-62
16. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, Osteoporosis prevention, diagnosis, and therapy: JAMA, 2001; 285; 785-95
17. Butin S, Griffoul I, Espitalier F, High incidence of vertebral osteoporotic fracture within the first year after liver transplantation: Clin Exp Rheumatol, 2017; 35; 913-18
18. Guichelaar MM, Kendall R, Malinchoc M, after OLT, Long-term follow-up and predictive factors: Liver Transpl, 2006; 12; 1390-402
19. Krol CG, Dekkers OM, Kroon HM, No association between BMD and prevalent vertebral fractures in liver transplant recipients at time of screening before transplantation: J Clin Endocrinol Metab, 2014; 99; 3677-85
20. Hardinger KL, Ho B, Schnitzler MA, Serial measurements of bone density at the lumbar spine do not predict fracture risk after liver transplantation: Liver Transpl, 2003; 9; 857-62
21. Librizzi MS, Guadalix S, Martínez-Díaz Guerra G, Trabecular bone score in patients with liver transplants after 1 year of risedronate treatment: Transpl Int, 2016; 29; 331-37
22. Brunova J, Kratochvilova S, Stepankova , Osteoporosis therapy with denosumab in organ transplant recipients: Front Endocrinol (Lausanne), 2018; 9; 162
23. Kaemmerer D, Lehmann G, Wolf G, Treatment of osteoporosis after liver transplantation with ibandronate: Transpl Int, 2010; 23; 753-59
24. Lan GB, Xie XB, Peng LK, Pathogenesis and management: Biomed Res Int, 2015; 2015; 413169
25. Holm JP, Hyldstrup L, Jensen JB, Time trends in osteoporosis risk factor profiles: A comparative analysis of risk factors, comorbidities, and medications over twelve years: Endocrine, 2016; 54; 241-55
26. González-Reimers E, Quintero-Platt G, Rodríguez-Rodríguez E, Bone changes in alcoholic liver disease: World J Hepatol, 2015; 7; 1258-64
27. Bouillon R, Okamura WH, Norman AW, Structure-function relationships in the vitamin D endocrine system: Endocr Rev, 1995; 16; 200-57
28. Baker AR, McDonnell DP, Hughes M, Cloning and expression of full-length cDNA encoding human vitamin D receptor: Proc Natl Acad Sci USA, 1988; 85; 329-84
29. Morrison NA, Qi JC, Tokita A, Prediction of bone density from vitamin D receptor alleles: Nature, 1994; 20; 284-87
30. Spector TD, Keen RW, Arden NK, Influence of vitamin D receptor genotype on bone mineral density in postmenopausal women: A twin study in Britain: BMJ, 1995; 27; 1357-60
31. Tokita A, Matsumoto H, Morrison NA, Vitamin D receptor alleles, bone mineral density and turnover in premenopausal Japanese women: J Bone Miner Res, 1996; 11; 1003-9
32. Arai H, Miyamoto K, Taketani Y, A vitamin D receptor gene polymorphism in the translation initiation codon: Effect on protein activity and relation to bone mineral density in Japanese women: J Bone Miner Res, 1997; 12; 915-21
33. Qin G, Dong Z, Zeng P, Association of vitamin D receptor BsmI gene polymorphism with risk of osteoporosis: A meta-analysis of 41 studies: Mol Biol Rep, 2013; 40; 497-506
34. Zhao B, Zhang W, Du S, Vitamin D receptor BsmI polymorphism and osteoporosis risk in post-menopausal women: Arch Med Sci, 2016; 12; 25-30
35. Zhang L, Yin X, Wang J, Associations between VDR gene polymorphisms and osteoporosis risk and bone mineral density in postmenopausal women: A systematic review and meta-analysis: Sci Rep, 2018; 8; 981
36. Fang Y, Rivadeneira F, van Meurs JB, Vitamin D receptor gene BsmI and TaqI polymorphisms and fracture risk: A meta-analysis: Bone, 2006; 39; 938-45
37. Uitterlinden AG, Ralston SH, Brandi ML, The association between common vitamin D receptor gene variations and osteoporosis: A participant-level meta-analysis: Ann Intern Med, 2006; 145; 255-64
38. Uitterlinden AG, Fang Y, Van Meurs JB, Genetics and biology of vitamin D receptor polymorphisms: Gene, 2004; 338; 143-56
39. Yamagata Z, Miyamura T, Iijima S, Vitamin D receptor gene polymorphism and bone mineral density in healthy Japanese women: Lancet, 1994; 344; 1027
40. Tokita A, Matsumoto H, Morrison NA, Vitamin D receptor alleles, bone mineral density and turnover in premenopausal Japanese women: J Bone Miner Res, 1996; 11; 1003-9
41. Ho YV, Briganti EM, Duan Y, Polymorphism of the vitamin D receptor gene and corticosteroid-related osteoporosis: Osteoporos Int, 1999; 9; 134-38
42. Matsuyama T, Ishii S, Tokita A, Vitamin D receptor genotypes and bone mineral density: Lancet, 1995; 345; 1238-39
43. Dawson-Hughes B, Harris SS, Finneran S, Calcium absorption on high and low calcium intakes in relation to vitamin D receptor genotype: J Clin Endocrinol Metab, 1995; 80; 3657-61
44. Kiel DP, Myers RH, Cupples LA, The BsmI vitamin D receptor restriction fragment length polymorphism (bb) influences the effect of calcium intake on bone mineral density: J Bone Miner Res, 1997; 12; 1049-57
45. Booth SL, Martini L, Peterson JW, Dietary phylloquinone depletion and repletion in older women: J Nutr, 2003; 133; 2565-69
46. Sergeev IN, Norman AW, Vitamin K-dependent gamma-carboxylation of the 1,25-dihydroxyvitamin D3 receptor: Biochem Biophys Res Commun, 1992; 189; 1543-47
47. Ozel L, Ata P, Ozel MS, Risk factors for osteoporosis after renal transplantation and effect of vitamin D receptor Bsm I polymorphism: Transplant Proc, 2011; 43; 858-62
Figures
 Figure 1. Both bone mineral density and single-nucleotide polymorphisms were measured in 27 patients. For Fok1, Apa1, and Taq1, there were no differences related to osteoporosis. However, there was a significant difference in incidence of osteoporosis associated with the VDR (Bsm1) GG group. * P=0.021.
Figure 1. Both bone mineral density and single-nucleotide polymorphisms were measured in 27 patients. For Fok1, Apa1, and Taq1, there were no differences related to osteoporosis. However, there was a significant difference in incidence of osteoporosis associated with the VDR (Bsm1) GG group. * P=0.021. Figure 2. Both bone mineral density and single-nucleotide polymorphisms (SNPs) were measured in 27 patients. There were no differences related to fragility fracture for any of the SNPs.
Figure 2. Both bone mineral density and single-nucleotide polymorphisms (SNPs) were measured in 27 patients. There were no differences related to fragility fracture for any of the SNPs. Tables
 Table 1. Primers and restriction enzymes used in the study.
Table 1. Primers and restriction enzymes used in the study. Table 2. Characteristics of the patients in whom bone mineral density was assessed (n=45).
Table 2. Characteristics of the patients in whom bone mineral density was assessed (n=45). Table 3. Relationship between bone mineral density and fragility fracture. There was a significant difference in frontal or lateral T-scores and young adult mean between the patients with and without fragility fractures.
Table 3. Relationship between bone mineral density and fragility fracture. There was a significant difference in frontal or lateral T-scores and young adult mean between the patients with and without fragility fractures. Table 4. There were no significant differences between the groups with and without osteoporosis in bone metabolism marker abnormalities, sex, presence or absence of menopause, body mass index, hepatitis C virus-related disease, or duration of administration of steroids or diuretics. A difference was found in the incidence of fragility fracture due to osteoporosis only in the patients with alcoholic liver cirrhosis.
Table 4. There were no significant differences between the groups with and without osteoporosis in bone metabolism marker abnormalities, sex, presence or absence of menopause, body mass index, hepatitis C virus-related disease, or duration of administration of steroids or diuretics. A difference was found in the incidence of fragility fracture due to osteoporosis only in the patients with alcoholic liver cirrhosis. Table 5. There were no significant differences between the groups with and without fragility fractures in bone metabolism marker abnormalities, sex, presence or absence of menopause, body mass index, hepatitis C virus-related disease, alcohol-related disease, or duration of administration of steroids or diuretics.
Table 5. There were no significant differences between the groups with and without fragility fractures in bone metabolism marker abnormalities, sex, presence or absence of menopause, body mass index, hepatitis C virus-related disease, alcohol-related disease, or duration of administration of steroids or diuretics. Table 1. Primers and restriction enzymes used in the study.
Table 1. Primers and restriction enzymes used in the study. Table 2. Characteristics of the patients in whom bone mineral density was assessed (n=45).
Table 2. Characteristics of the patients in whom bone mineral density was assessed (n=45). Table 3. Relationship between bone mineral density and fragility fracture. There was a significant difference in frontal or lateral T-scores and young adult mean between the patients with and without fragility fractures.
Table 3. Relationship between bone mineral density and fragility fracture. There was a significant difference in frontal or lateral T-scores and young adult mean between the patients with and without fragility fractures. Table 4. There were no significant differences between the groups with and without osteoporosis in bone metabolism marker abnormalities, sex, presence or absence of menopause, body mass index, hepatitis C virus-related disease, or duration of administration of steroids or diuretics. A difference was found in the incidence of fragility fracture due to osteoporosis only in the patients with alcoholic liver cirrhosis.
Table 4. There were no significant differences between the groups with and without osteoporosis in bone metabolism marker abnormalities, sex, presence or absence of menopause, body mass index, hepatitis C virus-related disease, or duration of administration of steroids or diuretics. A difference was found in the incidence of fragility fracture due to osteoporosis only in the patients with alcoholic liver cirrhosis. Table 5. There were no significant differences between the groups with and without fragility fractures in bone metabolism marker abnormalities, sex, presence or absence of menopause, body mass index, hepatitis C virus-related disease, alcohol-related disease, or duration of administration of steroids or diuretics.
Table 5. There were no significant differences between the groups with and without fragility fractures in bone metabolism marker abnormalities, sex, presence or absence of menopause, body mass index, hepatitis C virus-related disease, alcohol-related disease, or duration of administration of steroids or diuretics. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








