19 November 2021: Original Paper
Diagnostic Utility of Presepsin in Infections After Liver Transplantation: A Preliminary Study
Takahiro Yokose1ABCDEF, Masashi Takeuchi1ABCDEF, Hideaki Obara1ACDEFG*, Masahiro Shinoda2ADF, Hirofumi Kawakubo1ADF, Minoru KitagoDOI: 10.12659/AOT.933774
Ann Transplant 2021; 26:e933774
Abstract
BACKGROUND: Infectious complications after solid organ transplantation can be fatal, and early diagnosis and intervention are important. To the best of our knowledge, no study has examined the diagnostic utility of presepsin, a known accurate biomarker, for infectious complications after liver transplantation. This study aimed to evaluate the utility of presepsin for detecting infection and perioperative kinetics of presepsin after liver transplantation.
MATERIAL AND METHODS: This single-institutional prospective, observational study included 13 patients who underwent living-donor or deceased-donor liver transplantation. Perioperative serum presepsin level was measured 6 times within a week to evaluate its association with infectious complications and compare it with procalcitonin and C-reactive protein levels and leukocyte count. Postoperatively, patients were followed up for 15 days for infectious complications.
RESULTS: Five of the 13 patients developed infectious complications after liver transplantation. The median time for infection diagnosis was 9 postoperative days (25th-75th percentile, 7-10). Presepsin levels on 5 and 7 postoperative days were significantly higher in patients with infection than in those without (P=0.019 and P=0.011, respectively). In receiver operating characteristic analysis, area under the curve values of presepsin on 5 and 7 postoperative days (0.881 and 0.905, respectively) were higher than those of other biomarkers. The optimal cut-off value of presepsin was 1361 pg/mL on postoperative day 5 and 1375 pg/mL on postoperative day 7.
CONCLUSIONS: Although this study included a small number of patients, presepsin levels on postoperative days 5 and 7 may be useful indicators for infectious complications after liver transplantation.
Keywords: Bacterial Infections, C-Reactive Protein, Liver Transplantation, Presepsin Protein, Human, procalcitonin, Humans, Lipopolysaccharide Receptors, Living Donors, Peptide Fragments, Prospective Studies, ROC Curve, Sepsis
Background
Liver transplantation (LT) is a highly invasive type of abdominal surgery, and infections after LT can be fatal and require urgent therapeutic intervention [1–3]. Infectious complications have been reported to occur within the first 1–3 months after transplantation in 30–60% of reported cases [1–5]. Patients who undergo LT are often immunocompromised preoperatively due to liver cirrhosis and long-term administration of immunosuppressants for the treatment of primary diseases. Furthermore, immunosuppressant therapy continues after transplantation, resulting in the delayed presentation of infectious clinical symptoms. Therefore, to promptly manage infectious complications, early diagnosis and rapid treatment are essential.
LT triggers a strong inflammatory response due to a high degree of surgical stress, which frequently leads to non-infectious systemic inflammatory response syndrome (SIRS). Given this severe inflammatory response, conventional clinical symptoms, such as heart rate and body temperature, can mislead the diagnosis of postoperative infectious complications. Furthermore, corticosteroids, routinely administered as immunosuppressants postoperatively, suppress inflammatory cytokines and markers, resulting in further challenges in infection diagnosis. Several biomarkers, such as C-reactive protein (CRP), procalcitonin, and interleukin-6, are used to diagnose infections after LT [6–8]; however, even with these biomarkers, infections cannot be readily diagnosed, and differentiation from acute cellular rejection requiring treatment with immunosuppressant often becomes challenging.
Presepsin (soluble CD14 subtype) is a 13-kDa protein, which is a receptor for lipopolysaccharide-binding protein complexes and is produced when monocytes phagocytose bacteria [9], but its level does not increase in viral or fungal infections [10,11]. Presepsin is specific to bacterial infections and useful for distinguishing sepsis from non-infectious SIRS [12–14]. Compared with procalcitonin and CRP, presepsin is a superior diagnostic biomarker in terms of accuracy and detects infections in the earlier phase [12,13,15,16]. In patients with liver cirrhosis, presepsin level tends to increase with cirrhosis progression even without infection and is associated with survival rate [17]. Thus, the usefulness of presepsin for diagnosing infection in patients with liver cirrhosis who require LT is controversial [17,18]. Similarly, in patients with end-stage kidney disease, although presepsin level increases even without infection with disease progression, it decreases to normal levels after kidney transplantation [19]. However, in LT patients, the perioperative kinetics of presepsin level and its usefulness in the diagnosis of infections remain unknown.
This study aimed to determine an optimal indicator for detecting infectious complications by comparing presepsin with other conventional inflammatory indicators and to determine the diagnostic accuracy of presepsin in predicting infectious complications.
Material and Methods
PATIENTS:
This prospective observational single-institution study involved 13 consecutive patients who had undergone LT between October 2018 and July 2019 at Keio University Hospital (Tokyo, Japan). Fifteen patients aged ≥20 years who had undergone living-donor LT (LDLT) or deceased-donor LT (DDLT) at our hospital were included in this study. Two patients with a preoperative active bacterial infection, apart from those with viral hepatitis, were excluded from this study. Similarly, patients who declined to participate in this study were excluded. The study protocol was approved by the Human Experimentation Committee of our institution (No. 20180092) and was conducted in accordance with the Helsinki Declaration of 1975. Verbal informed consent was obtained from all patients.
BLOOD SAMPLES AND DATA COLLECTION:
To measure serum presepsin and procalcitonin levels, blood samples were collected 1 day before surgery or induction of anesthesia (baseline), immediately postoperatively (known as postoperative day [POD] 0), and POD 1, 2, 3, 5, and 7. Postoperatively, patients were followed up for 15 days to evaluate any infectious complications and kidney function.
Two-milliliter blood samples were collected in Venoject II tubes (Terumo, Tokyo, Japan) and processed immediately. To separate plasma from peripheral blood cells, blood samples were centrifuged at 1500×g at room temperature for 10 min within 4 h of collection. All plasma samples were stored at −80°C and thawed at room temperature before presepsin and procalcitonin analyses. Presepsin levels were measured using a fully automatic immunoassay analyzer (PATHFAST, LSI Medience, Tokyo, Japan) according to the manufacturer’s instructions, and the lower limit of detection was set to 20 pg/mL. Procalcitonin levels were measured using an electro-chemiluminescent immunoassay (LSI Medience, Tokyo, Japan), and the lower limit of detection was set to 0.02 ng/mL. CRP and leukocyte counts were measured at our hospital laboratory. CRP levels were measured using a latex agglutination immunoturbidimetry method, and the lower limit of detection was set to 0.02 mg/dL.
DEFINITION OF COMPLICATIONS:
Infectious complications were defined according to the International Sepsis Forum Consensus Conference criteria [20]. Sepsis and septic shock were defined according to the Third International Consensus Definitions for Sepsis and Septic Shock [21]. Pneumonia was defined as the presence of both bacterial growth from sputum and abnormal radiographic infiltrate. Bloodstream infection was defined as bacterial growth from blood cultures caused by a microorganism not typically regarded as a common skin contaminant. Surgical-site infection was defined as the presence of clinical symptoms based on infection emerging from the surgical intervention site (superficial incisional, deep incisional, or involving organ space) [22]. Additionally, an intra-abdominal abscess, diagnosed using ultrasonography or computed tomography, the presence of a positive aspirate culture, or both were considered as a surgical-site infection [22]. Cholangitis was defined as the presence of either fever or elevated serum liver enzymes, as well as a positive bile culture and the absence of other infection markers, such as abscess formation, as determined using ultrasound or computed tomography. Acute kidney injury (AKI) was defined according to the Kidney Disease Improving Global Outcomes criteria [23].
SURGICAL PROCEDURES AND PERIOPERATIVE PROTOCOLS:
Surgical procedures used to perform LT have been previously described [24–27]. Splenectomy was simultaneously performed in ABO-incompatible patients or hepatitis C virus-positive patients. The regimen for immunosuppressants has been previously described [27]. For all patients, the regimen included administration of calcineurin inhibitor, steroids, and anti-metabolites. For ABO-incompatible patients, treatment included preoperative administration of calcineurin inhibitor, steroids, and anti-metabolites. Rituximab was administered once or twice preoperatively to address B-cell depletion.
Antibiotics, including penicillin and cephem, were administered prophylactically to prevent bacterial infection [27]. Prophylactic treatments were usually discontinued the day following extubation. We performed surveillance cultures twice a week for 1 month after transplantation, even if there were no signs of infection. We also obtained cultures when clinical symptoms of suspected infection such as fever appeared. When a patient with a positive bacterial culture developed high fever, appropriate antibiotics were selected based on the results of a sensitivity test and were administered until infection control was achieved.
STATISTICAL ANALYSIS:
Statistical analysis was conducted using IBM SPSS Statistics for Macintosh, version 25.0 (IBM Corp., Armonk, NY, USA). Continuous variables were compared using the Mann-Whitney U-test. The best indicator for predicting infectious complications was determined using the receiver operating characteristic (ROC) curve and area under the curve (AUC). The optimal cut-off values for each biomarker predicting infectious complications were examined by analyzing the ROC curve. Continuous variables are summarized by medians (25th–75th percentiles), whereas categorical variables are summarized by frequency and proportion (%). All statistical tests were two-tailed, and a
Results
PATIENT CHARACTERISTICS:
A total of 13 patients were included in this study (median age, 44 years [25th–75th percentiles, 38–55 years]; males, n=4). The primary diseases were alcohol-related liver disease (n=4 patients), primary biliary cholangitis (n=3 patients), primary sclerosing cholangitis (n=2 patients), autoimmune hepatitis (n=2 patients), hepatopulmonary syndrome (n=1 patient), and unknown (n=1 patient). LDLT and DDLT were performed in 7 and 6 patients, respectively. Patient clinical and perioperative characteristics are shown in Table 1.
COMPLICATIONS:
Among the 13 patients, infectious complications occurred in 5 (38.5%) patients (infection group). The median duration for diagnosis of an infectious complication after LT was 9 days (25th–75th percentiles, 7–10 days). In the infection group, 2 patients developed a bloodstream infection, 1 patient was diagnosed with intra-abdominal abscess, 1 patient was diagnosed with cholangitis, and 1 patient was diagnosed with pneumonia. One of the 2 patients who developed a bloodstream infection was diagnosed with sepsis. No patients were diagnosed with septic shock. All postoperative infectious complications and cultured microorganisms are shown in Table 2.
Postoperative AKI occurred in 9 patients and required temporary continuous renal replacement therapy (CRRT) in 4 patients (Table 1). All of the 5 patients who developed infectious complications had postoperative AKI, and 3 patients required temporary CRRT.
PERIOPERATIVE VARIABLES:
Biomarker levels at each time point were compared between the 2 groups (Figure 1, Table 3). Presepsin levels had a postoperative peak from immediately after surgery to POD 1, which decreased after POD 2 in the non-infection group but tended to increase after POD 2 in the infection group (Figure 1A). Presepsin levels on POD 5 and 7 were significantly higher in the infection group than in the non-infection group (P=0.019 and P=0.011, respectively) (Figure 1A, Table 3). Presepsin levels after the peak were higher in patients with AKI than in those without AKI (Supplementary Figure 1A). In patients with AKI, presepsin levels on POD 5 and POD 7 were higher in the infection group than in the non-infection group (P=0.190 and P=0.111, respectively). Procalcitonin levels peaked on POD 2, and the peak value was higher in the infection group than in the non-infection group (Figure 1B, Table 3). Procalcitonin levels after the peak were higher in patients with AKI than in those without AKI (Supplementary Figure 1B); however, in patients with AKI, there were no differences between the infected and the non-infected groups. CRP levels peaked from POD 1 to 2, and the peak value was higher in the infection group than in the non-infection group (Figure 1C, Table 3). Leukocytes tended to peak between POD 2 and 3, but no significant difference was noted between the groups (Figure 1D).
The perioperative kinetics of each marker in all 13 patients were obtained for the infection and non-infection groups (Figure 2). In 1 patient who developed bloodstream infection on POD 2, presepsin level increased from POD 1 to 2 and subsequently decreased by POD 7 (Figure 2A). Meanwhile, procalcitonin and CRP levels peaked at POD 2 as in the other patients in whom it was difficult to distinguish infection from non-infectious SIRS (Figure 2B, 2C). Leukocytes did not increase at each time point (Figure 2D). In 1 patient with infection on POD 7, only presepsin level was elevated on POD 7 (Figure 2A), but the other 3 marker levels remained unchanged (Figure 2B–2D). In patients without infectious complications, presepsin level did not increase significantly after surgery (Figure 2A); however, procalcitonin and CRP levels peaked around POD 2, which was similar to those with infectious complications (Figure 2B, 2C).
When the AUC values of presepsin, procalcitonin, and CRP levels and leukocyte count on POD 5 and 7 were compared, the values of presepsin on POD 5 and POD 7 were higher than those of the other 3 biomarkers (Figure 3A, 3B). In particular, the AUC value of presepsin on POD 5 was 0.881 and on POD 7 it was 0.905. The optimal cut-off values for each biomarker predicting infectious complications are shown in Table 4. The cut-off value of presepsin was 1361 pg/mL (100% sensitivity, 86% specificity) on POD 5 and 1375 pg/mL (100% sensitivity, 86% specificity) on POD 7. In patients with AKI, the AUC values of presepsin on POD 5 and POD 7 (0.800 and 0.850, respectively) were higher than procalcitonin values (0.600 and 0.500, respectively).
COMPARISON OF PRESEPSIN LEVEL AT BASELINE:
In 5 (38.5%) of the patients, presepsin level at baseline was higher than the cut-off value in severe sepsis (1000 pg/mL) [28], even though patients with preoperative bacterial infections were excluded. Therefore, we included these 5 patients in the preoperative high group and the remaining 8 patients in the preoperative low group. Patient characteristics of the 2 groups were compared (Table 5). Model for end-stage liver disease (MELD) score and Eastern Cooperative Oncology Group performance status (ECOG-PS) were significantly higher in the preoperative high group than in the preoperative low group (P=0.011 and P=0.024, respectively).
Presepsin levels at each time point were compared between patients with and without infection in both groups (Figure 4). Among the 5 patients in the preoperative high group, infectious complications occurred in 2 (40.0%) patients. In the preoperative high group, presepsin level remained high after LT in the infection group but tended to decrease in the non-infection group (Figure 4A). In the preoperative low group, presepsin level decreased after it peaked at POD 0 in the non-infection group, but increased after POD 2 in the infection group, and was significantly higher at POD 3, 5, and 7 (P=0.036, P=0.036, and P=0.036, respectively) than those in the non-infected group (Figure 4B).
Discussion
This study found that serum presepsin levels on POD 5 and 7 may be more useful indicators compared to procalcitonin and CRP levels and leukocyte count in detecting infectious complications after LT. Although some patients with liver cirrhosis who needed LT had high preoperative presepsin levels, the levels generally decrease postoperatively in patients without infections. In addition, if preoperative presepsin levels are low, it will be significantly higher from POD 3 to 7 in patients with postoperative infectious complications than in those without.
To date, several studies have shown an association between presepsin levels and postoperative infectious complications in various types of surgeries [29–32], including heart transplantation [33] and allogeneic hematopoietic cell transplantation [10]. However, to the best of our knowledge, this is the first preliminary study demonstrating the usefulness of presepsin in detecting infectious complications after LT.
In this study, each biomarker level was measured 7 times preoperatively and until POD 7, and presepsin levels were compared with 3 other biomarkers (procalcitonin, CRP, and leukocyte counts) that have conventionally been used as diagnostic markers for infection. Our study showed that presepsin levels on POD 5 and 7 were significantly associated with infectious complications. This finding supports those of previous studies that reported that gastrointestinal anastomotic leakage or surgical-site infection could be detected through upregulation of presepsin levels on POD 5 or POD 7 [29,31,32]. In our study, infectious complications occurred after a median of 9 days. This outcome was in accordance with a previous study that found that bacteremia after LT occurred within a median of 12 days [2] and that most infectious complications after abdominal surgery occur within 4–30 days postoperatively [34].
In the present study, patients with preoperative bacterial infections were excluded; however, 38% of patients had elevated preoperative presepsin levels with significantly poorer ECOG-PS and higher MELD scores. Presepsin level in the liver may have been elevated without infection due to inflammation and fibrosis from underlying diseases [35,36]. Furthermore, presepsin level tends to increase with cirrhosis progression and renal dysfunction, even without infections [17,18,37]. Therefore, the reason for the increased level may be cirrhosis progression, hepatorenal syndrome, or potential inflammation. However, as shown in our study, presepsin levels generally decrease after POD 2 in patients without infection despite high preoperative levels. This study result will provide meaningful suggestions for patient selection and target number of cases when conducting similar studies on a larger scale in the future.
AKI occurs frequently after liver transplantation due to instability of fluid balance, and the use of many drugs that cause renal damage [3,25]. In a study conducted on patients in the intensive care unit, although presepsin and procalcitonin were affected by renal function, both were useful biomarkers in detecting sepsis, even in patients with AKI [37]. In our study, although postoperative AKI occurred in 69% of patients, presepsin levels of POD 5 and POD 7 were higher in the infection group than in the non-infection group, even in patients with AKI. There was no association between CRRT and levels of presepsin (data not shown). In our study, although presepsin was affected by renal function, presepsin may help in the diagnosis of infectious complications after liver transplantation, which frequently leads to AKI; however, that of prognostic utility of procalcitonin was unclear.
In this study, infection occurred mostly from POD 7 to 10. As previously reported, presepsin level increases from an earlier time point of infection, but procalcitonin and CRP levels increase after a couple of days after onset [12,15,16], which may have caused differences in AUC on POD 5 and 7. In addition, presepsin may be useful for the early detection of infectious complications, as its level increases from POD 0 to POD 1 and peaks earlier than other biomarkers. According to Surviving Sepsis Campaign recommendations, initial fluid loading and administration of antimicrobials immediately are extremely important in helping patients survive sepsis [38]. Measurement of presepsin levels can lead to an earlier diagnosis of infection and more effective treatment.
This study had several limitations. First, it had a small sample size and the results are very preliminary; thus, future studies with a larger number of cases at multiple centers are needed. Second, this single-institution, prospective consecutive registration study was undertaken in Japan and exclusively targeted the Japanese population, which may have resulted in potential selection bias. Finally, the relationship between the severity of infectious complications and presepsin was unclear due to the small number of cases of sepsis or septic shock; hence, further studies are needed to clarify this relationship.
Conclusions
Presepsin levels on POD 5 and 7 may be useful indicators for detecting infectious complications after LT compared with procalcitonin and CRP level and leukocyte count. Although some patients with liver cirrhosis showed increased presepsin levels without infection, serum presepsin levels decrease after LT and may help divide patients precisely into those with infection and without.
Figures
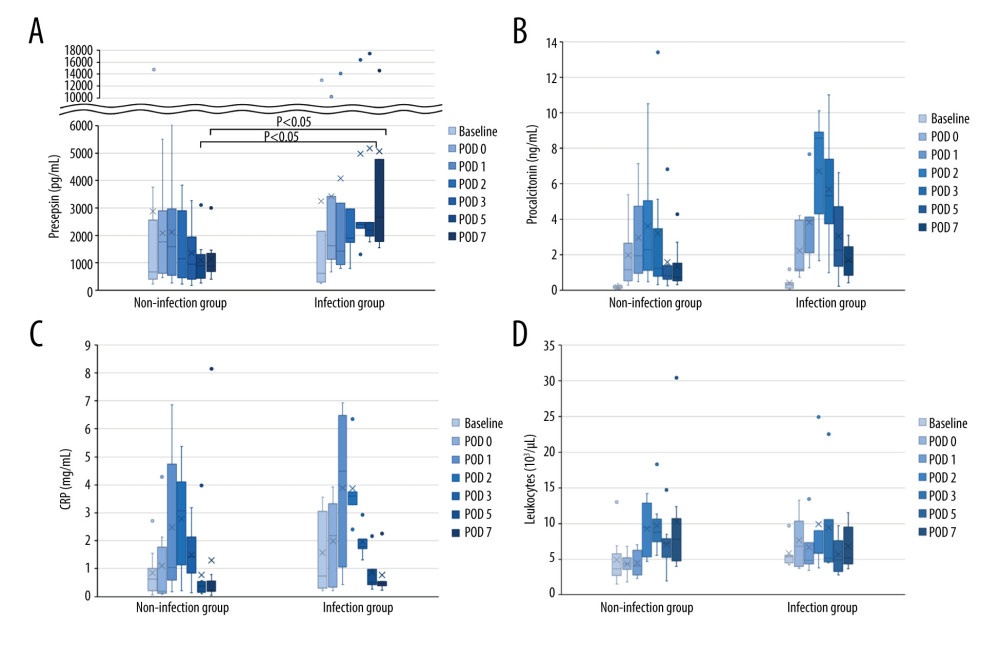 Figure 1. Perioperative change of 4 biomarkers in patients undergoing liver transplantation(A) Presepsin; (B) Procalcitonin; (C) C-reactive protein (CRP); (D) Leukocyte counts. Lines indicate median values. The box plot indicates 25th–75th percentiles. The left side indicates patients with non-infectious complications, and the right side indicates patients with infectious complications within 15 days postoperatively. (Microsoft Excel for Macintosh, version 16.16.27, Microsoft).
Figure 1. Perioperative change of 4 biomarkers in patients undergoing liver transplantation(A) Presepsin; (B) Procalcitonin; (C) C-reactive protein (CRP); (D) Leukocyte counts. Lines indicate median values. The box plot indicates 25th–75th percentiles. The left side indicates patients with non-infectious complications, and the right side indicates patients with infectious complications within 15 days postoperatively. (Microsoft Excel for Macintosh, version 16.16.27, Microsoft). 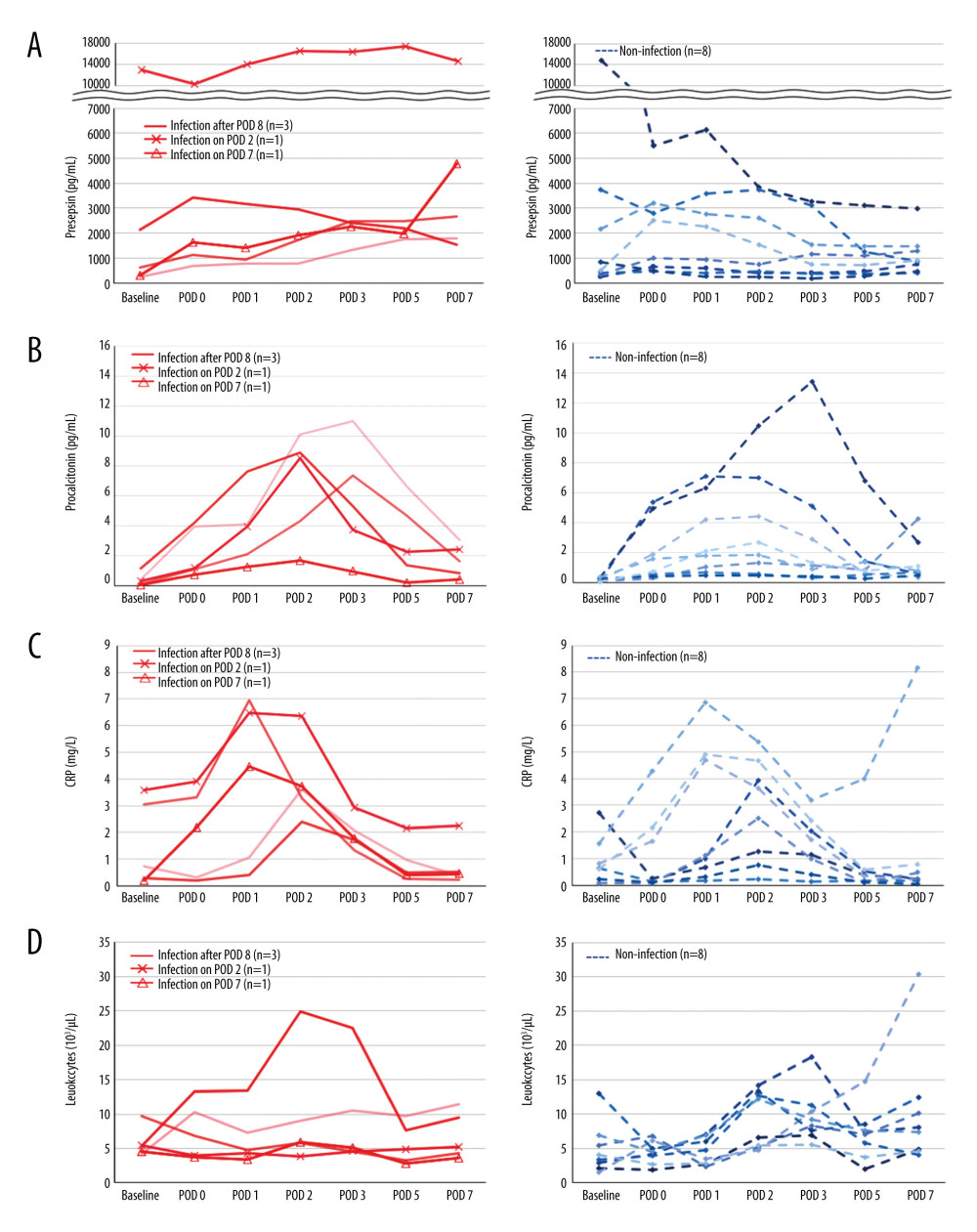 Figure 2. The perioperative kinetics of each marker in all 13 patients(A) Presepsin; (B) Procalcitonin; (C) C-reactive protein (CRP); (D) Leukocyte counts. Left side indicates infection group. Infection occurring after POD 8 (n=3), on POD 2 (n=1), and on POD 7 (n=1) are reported separately. Right side indicates non-infection group. (Microsoft Excel for Macintosh, version 16.16.27, Microsoft).
Figure 2. The perioperative kinetics of each marker in all 13 patients(A) Presepsin; (B) Procalcitonin; (C) C-reactive protein (CRP); (D) Leukocyte counts. Left side indicates infection group. Infection occurring after POD 8 (n=3), on POD 2 (n=1), and on POD 7 (n=1) are reported separately. Right side indicates non-infection group. (Microsoft Excel for Macintosh, version 16.16.27, Microsoft). 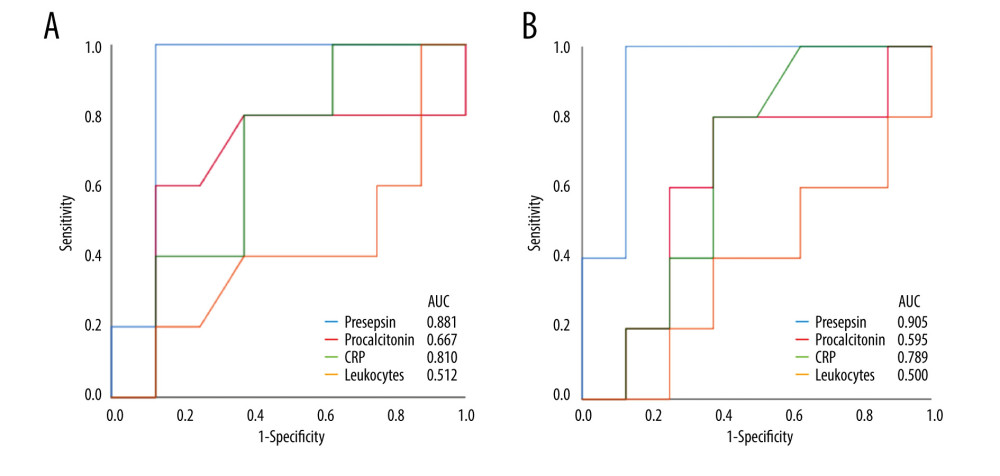 Figure 3. Receiver operating characteristic curve of presepsin, procalcitonin, and C-reactive protein (CRP) levels and leukocyte count used to determine infectious complications(A) Postoperative day 5; (B) Postoperative day 7. (IBM SPSS Statistics for Macintosh, version 25.0 IBM Corp.).
Figure 3. Receiver operating characteristic curve of presepsin, procalcitonin, and C-reactive protein (CRP) levels and leukocyte count used to determine infectious complications(A) Postoperative day 5; (B) Postoperative day 7. (IBM SPSS Statistics for Macintosh, version 25.0 IBM Corp.). 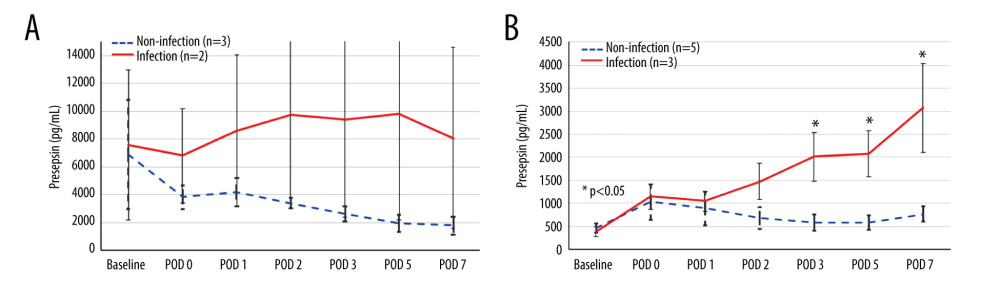 Figure 4. The perioperative kinetics of presepsin levels compared between patients with and without infection in the preoperative high and low groups(A) Preoperative high presepsin level group (n=5); (B) Preoperative low presepsin level group (n=8). The dotted line indicates patients without infectious complications, and the solid line indicates patients with infectious complications within 15 days postoperatively. An error bar shows the standard error of the mean. (Microsoft Excel for Macintosh, version 16.16.27, Microsoft).
Figure 4. The perioperative kinetics of presepsin levels compared between patients with and without infection in the preoperative high and low groups(A) Preoperative high presepsin level group (n=5); (B) Preoperative low presepsin level group (n=8). The dotted line indicates patients without infectious complications, and the solid line indicates patients with infectious complications within 15 days postoperatively. An error bar shows the standard error of the mean. (Microsoft Excel for Macintosh, version 16.16.27, Microsoft). Tables
Table 1. Patient characteristics.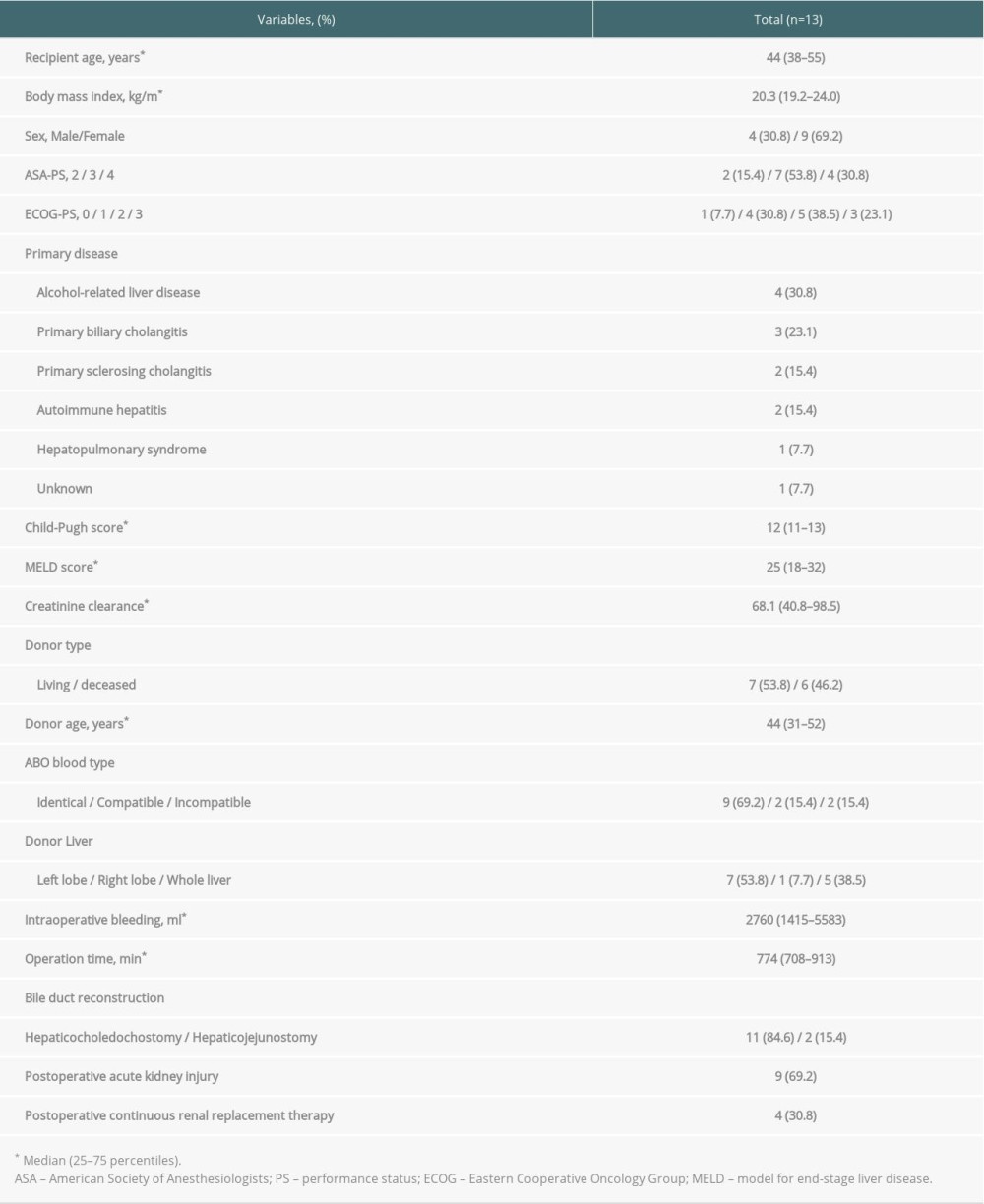 Table 2. Types of infectious complications.
Table 2. Types of infectious complications.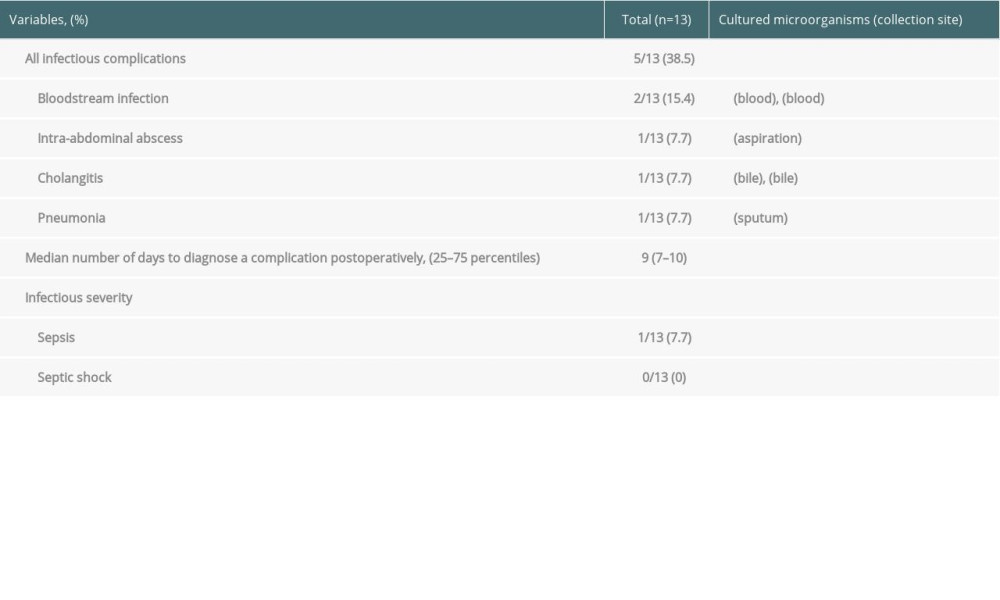 Table 3. Comparison of changes in presepsin, procalcitonin, and C-reactive protein levels and leukocyte count between the infection and non-infection groups.
Table 3. Comparison of changes in presepsin, procalcitonin, and C-reactive protein levels and leukocyte count between the infection and non-infection groups.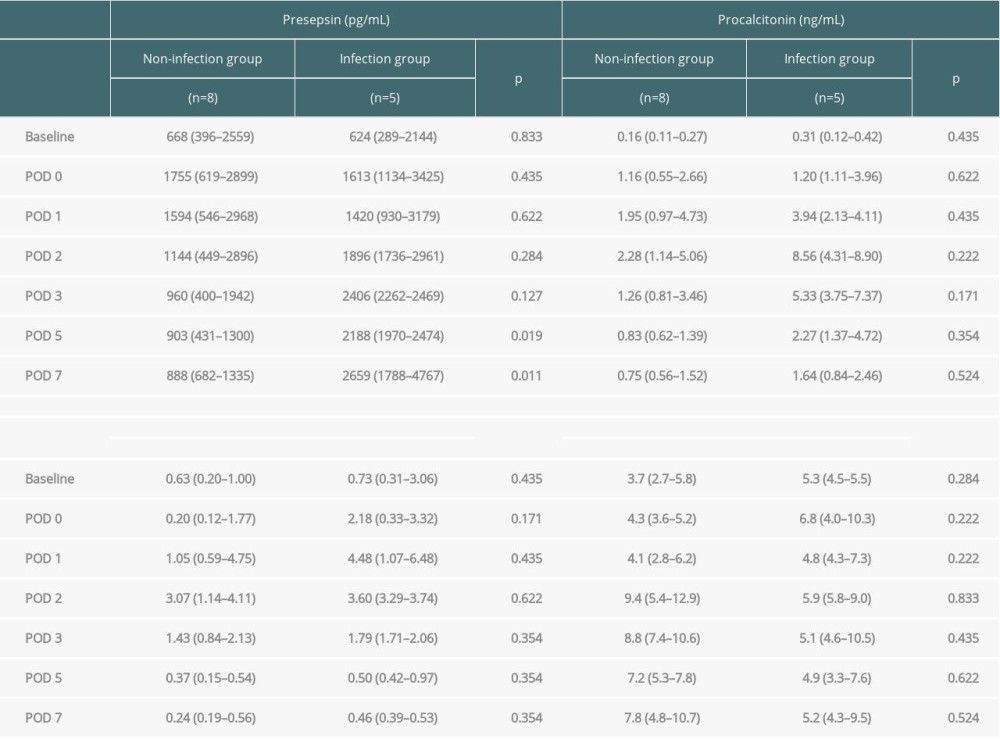 Table 4. Diagnostic accuracy of presepsin, procalcitonin, and CRP levels and leukocyte counts in terms of infectious complications on POD 5 and 7.
Table 4. Diagnostic accuracy of presepsin, procalcitonin, and CRP levels and leukocyte counts in terms of infectious complications on POD 5 and 7.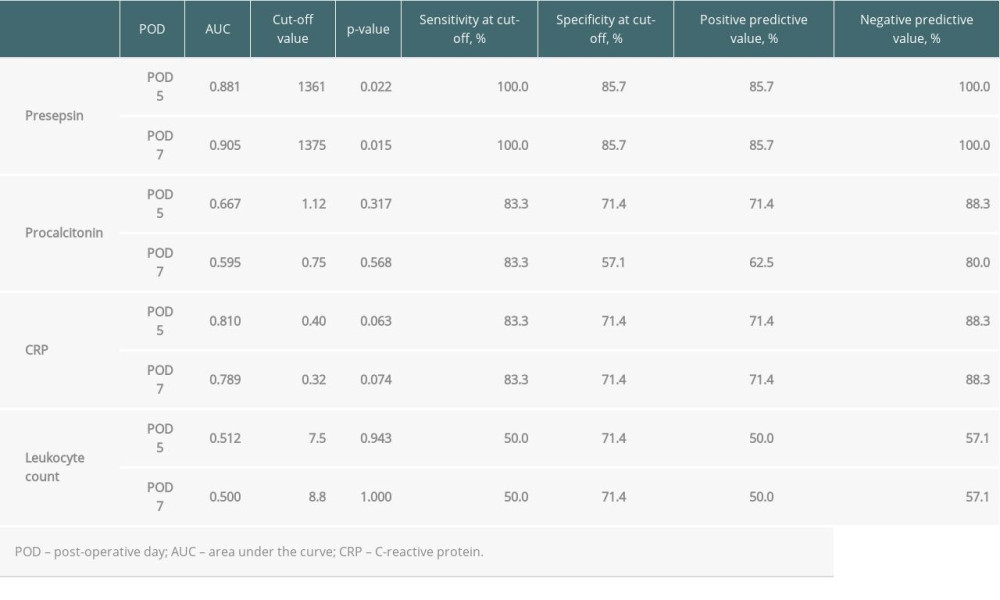 Table 5. Comparison of patient characteristics between high and low preoperative levels of presepsin.
Table 5. Comparison of patient characteristics between high and low preoperative levels of presepsin.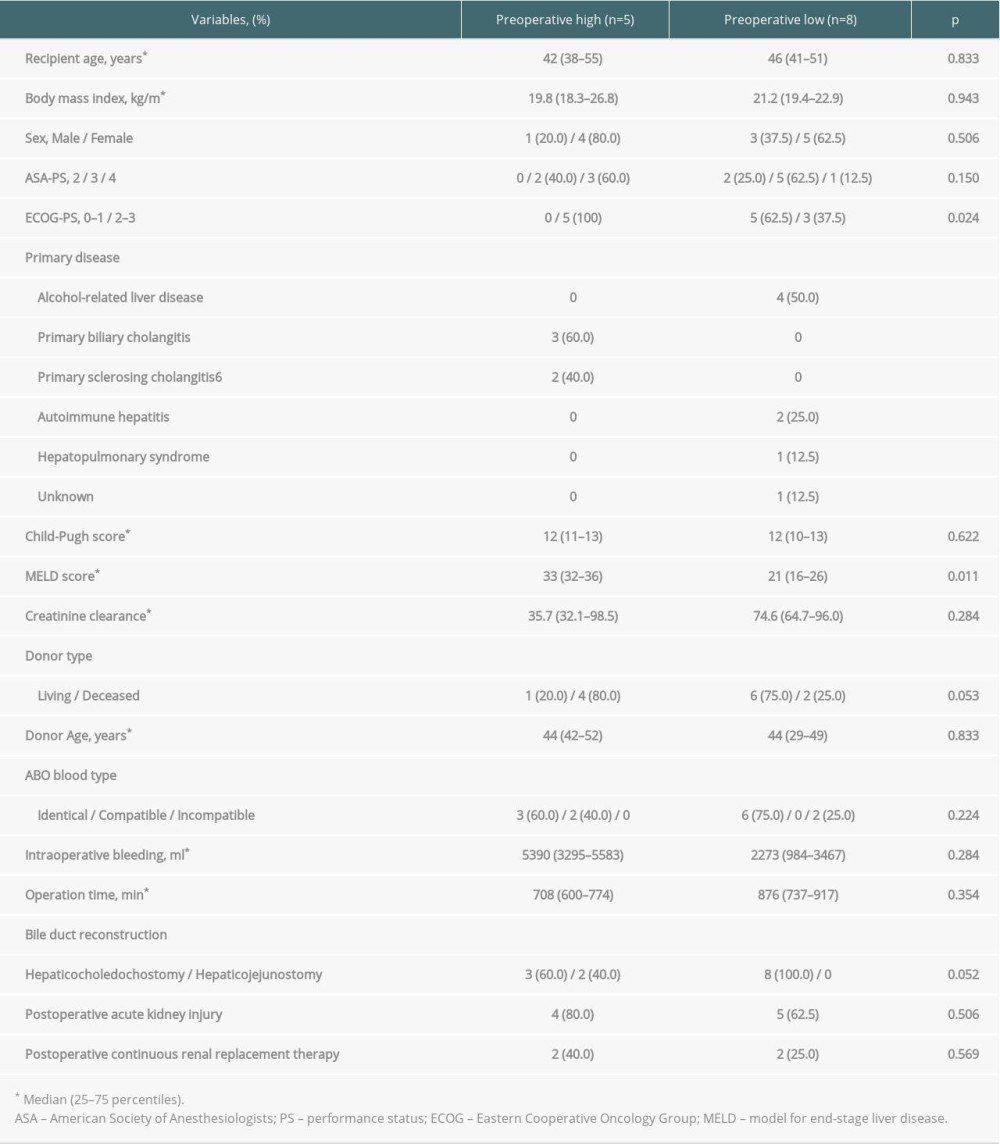
References
1. Hellinger WC, Crook JE, Heckman MG, Surgical site infection after liver transplantation: Risk factors and association with graft loss or death: Transplantation, 2009; 87; 1387-93
2. Iida T, Kaido T, Yagi S, Posttransplant bacteremia in adult living donor liver transplant recipients: Liver Transpl, 2010; 16; 1379-85
3. Marubashi S, Ichihara N, Kakeji Y, “Real-time” risk models of postoperative morbidity and mortality for liver transplants: Ann Gastroenterol Surg, 2018; 3; 75-95
4. Bellier C, Bert F, Durand F, Risk factors for Enterobacteriaceae bacteremia after liver transplantation: Transpl Int, 2008; 21; 755-63
5. Soong RS, Chan KM, Chou HS, The risk factors for early infection in adult living donor liver transplantation recipients: Transplant Proc, 2012; 44; 784-86
6. Perrakis A, Yedibela S, Schellerer V, Procalcitonin in the setting of complicated postoperative course after liver transplantation: Transplant Proc, 2010; 42; 4187-90
7. Kaido T, Ogawa K, Fujimoto Y, Perioperative changes of procalcitonin levels in patients undergoing liver transplantation: Transpl Infect Dis, 2014; 16; 790-96
8. Gür A, Oguzturk H, Köse A, Prognostic value of procalcitonin, CRP, serum amyloid A, lactate and IL-6 markers in liver transplant patients admitted to ED with suspected infection: In Vivo, 2017; 31; 1179-85
9. Shirakawa K, Naitou K, Hirose J, Presepsin (sCD14-ST): Development and evaluation of one-step ELISA with a new standard that is similar to the form of presepsin in septic patients: Clin Chem Lab Med, 2011; 49; 937-39
10. Arai Y, Mizugishi K, Nonomura K, Phagocytosis by human monocytes is required for the secretion of presepsin: J Infect Chemother, 2015; 21; 564-69
11. Stoma I, Karpov I, Uss A, Low levels of procalcitonin or presepsin combined with significantly elevated C-reactive protein may suggest an invasive fungal infection in hematological patients with febrile neutropenia: Hemasphere, 2019; 3; e170
12. Ebisawa K, Koya J, Nakazaki K, Usefulness of presepsin for early detection of infections in patients with hematologic disorders: Clin Chim Acta, 2018; 486; 374-80
13. Masson S, Caironi P, Fanizza C, Circulating presepsin (soluble CD14 subtype) as a marker of host response in patients with severe sepsis or septic shock: Data from the multicenter, randomized ALBIOS trial: Intensive Care Med, 2015; 41; 12-20
14. Ulla M, Pizzolato E, Lucchiari M, Diagnostic and prognostic value of presepsin in the management of sepsis in the emergency department: A multicenter prospective study: Crit Care, 2013; 17; R168
15. Koizumi Y, Shimizu K, Shigeta M, Plasma presepsin level is an early diagnostic marker of severe febrile neutropenia in hematologic malignancy patients: BMC Infect Dis, 2017; 17; 27
16. Koh H, Aimoto M, Katayama T, Diagnostic value of levels of presepsin (soluble CD14-subtype) in febrile neutropenia in patients with hematological disorders: J Infect Chemother, 2016; 22; 466-71
17. Ferrarese A, Frigo AC, Mion MM, Diagnostic and prognostic role of presepsin in patients with cirrhosis and bacterial infection: Clin Chem Lab Med, 2021; 59; 775-82
18. Novelli S, Morabito V, Ruberto F, Diagnostic value of presepsin for bacterial infection in cirrhosis: A pilot study: Transplant Proc, 2020; 52; 1593-600
19. Saito J, Hashiba E, Kushikata T, Changes in presepsin concentrations in surgical patients with end-stage kidney disease undergoing living kidney transplantation: A pilot study: J Anesth, 2016; 30; 174-77
20. Calandra T, Cohen JInternational Sepsis Forum Definition of Infection in the ICU Consensus Conference, The international sepsis forum consensus conference on definitions of infection in the intensive care unit: Crit Care Med, 2005; 33; 1538-48
21. Seymour CW, Liu VX, Iwashyna TJ, Assessment of clinical criteria for sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3): JAMA, 2016; 315; 762-74
22. National Healthcare Safety Network, Centers for Disease Control and Prevention: Surgical site infection (SSI) event [Published January 2020] Available on: https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf
23. Kellum JA, Lameire NKDIGO AKI Guideline Work Group, Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1): Crit Care, 2013; 17; 204
24. Kawachi S, Shimazu M, Wakabayashi G, Biliary complications in adult living-donor liver transplantation with duct-to-duct hepaticocholedochostomy or Roux-en-Y hepaticojejunostomy biliary reconstruction: Surgery, 2002; 132; 48-56
25. Tanabe M, Kawachi S, Obara H, Current progress in ABO-incompatible liver transplantation: Eur J Clin Invest, 2010; 40; 943-49
26. Kishida N, Shinoda M, Itano O, Increased incidence of thrombotic microangiopathy after ABO-incompatible living donor liver transplantation: Ann Transplant, 2016; 21; 755-64
27. Ei S, Shinoda M, Itano O, Effects of addition of early enteral nutritional support during the postoperative phase in patients after living-donor liver transplantation: Ann Transplant, 2015; 20; 357-65
28. Carpio R, Zapata J, Spanuth E, Hess G, Utility of presepsin (sCD14-ST) as a diagnostic and prognostic marker of sepsis in the Emergency Department: Clin Chim Acta, 2015; 450; 169-75
29. Cikot M, Kasapoglu P, Isiksacan N, The importance of presepsin value in detection of gastrointestinal anastomotic leak: A pilot study: J Surg Res, 2018; 228; 100-6
30. Saito J, Hashiba E, Mikami A, Pilot study of changes in presepsin concentrations compared with changes in procalcitonin and C-reactive protein concentrations after cardiovascular surgery: J Cardiothorac Vasc Anesth, 2017; 31; 1262-67
31. Koakutsu T, Sato T, Aizawa T, Postoperative changes in presepsin level and values predictive of surgical site infection after spinal surgery: A single-center, prospective observational study: Spine (Phila Pa 1976), 2018; 43; 578-84
32. Takeuchi M, Yokose T, Kawakubo H, The perioperative presepsin as an accurate diagnostic marker of postoperative infectious complications after esophagectomy: A prospective cohort study: Esophagus, 2020; 17; 399-407
33. Franeková J, Sečník P, Lavríková P, Serial measurement of presepsin, procalcitonin, and C-reactive protein in the early postoperative period and the response to antithymocyte globulin administration after heart transplantation: Clin Transplant, 2017; 31; ctr.12870
34. Thompson JS, Baxter BT, Allison JG, Temporal patterns of postoperative complications: Arch Surg, 2003; 138; 596-602
35. Sandler NG, Koh C, Roque A, Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection: Gastroenterology, 2011; 141; 1220-30
36. Ogawa Y, Imajo K, Yoneda M, Soluble CD14 levels reflect liver inflammation in patients with nonalcoholic steatohepatitis: PLoS One, 2013; 8; e65211
37. Nakamura Y, Hoshino K, Kiyomi F, Comparison of accuracy of presepsin and procalcitonin concentrations in diagnosing sepsis in patients with and without acute kidney injury: Clin Chim Acta, 2019; 490; 200-6
38. Rhodes A, Evans LE, Alhazzani W, Surviving sepsis campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016: Crit Care Med, 2017; 45; 486-552
Figures
 Figure 1. Perioperative change of 4 biomarkers in patients undergoing liver transplantation(A) Presepsin; (B) Procalcitonin; (C) C-reactive protein (CRP); (D) Leukocyte counts. Lines indicate median values. The box plot indicates 25th–75th percentiles. The left side indicates patients with non-infectious complications, and the right side indicates patients with infectious complications within 15 days postoperatively. (Microsoft Excel for Macintosh, version 16.16.27, Microsoft).
Figure 1. Perioperative change of 4 biomarkers in patients undergoing liver transplantation(A) Presepsin; (B) Procalcitonin; (C) C-reactive protein (CRP); (D) Leukocyte counts. Lines indicate median values. The box plot indicates 25th–75th percentiles. The left side indicates patients with non-infectious complications, and the right side indicates patients with infectious complications within 15 days postoperatively. (Microsoft Excel for Macintosh, version 16.16.27, Microsoft). Figure 2. The perioperative kinetics of each marker in all 13 patients(A) Presepsin; (B) Procalcitonin; (C) C-reactive protein (CRP); (D) Leukocyte counts. Left side indicates infection group. Infection occurring after POD 8 (n=3), on POD 2 (n=1), and on POD 7 (n=1) are reported separately. Right side indicates non-infection group. (Microsoft Excel for Macintosh, version 16.16.27, Microsoft).
Figure 2. The perioperative kinetics of each marker in all 13 patients(A) Presepsin; (B) Procalcitonin; (C) C-reactive protein (CRP); (D) Leukocyte counts. Left side indicates infection group. Infection occurring after POD 8 (n=3), on POD 2 (n=1), and on POD 7 (n=1) are reported separately. Right side indicates non-infection group. (Microsoft Excel for Macintosh, version 16.16.27, Microsoft). Figure 3. Receiver operating characteristic curve of presepsin, procalcitonin, and C-reactive protein (CRP) levels and leukocyte count used to determine infectious complications(A) Postoperative day 5; (B) Postoperative day 7. (IBM SPSS Statistics for Macintosh, version 25.0 IBM Corp.).
Figure 3. Receiver operating characteristic curve of presepsin, procalcitonin, and C-reactive protein (CRP) levels and leukocyte count used to determine infectious complications(A) Postoperative day 5; (B) Postoperative day 7. (IBM SPSS Statistics for Macintosh, version 25.0 IBM Corp.). Figure 4. The perioperative kinetics of presepsin levels compared between patients with and without infection in the preoperative high and low groups(A) Preoperative high presepsin level group (n=5); (B) Preoperative low presepsin level group (n=8). The dotted line indicates patients without infectious complications, and the solid line indicates patients with infectious complications within 15 days postoperatively. An error bar shows the standard error of the mean. (Microsoft Excel for Macintosh, version 16.16.27, Microsoft).
Figure 4. The perioperative kinetics of presepsin levels compared between patients with and without infection in the preoperative high and low groups(A) Preoperative high presepsin level group (n=5); (B) Preoperative low presepsin level group (n=8). The dotted line indicates patients without infectious complications, and the solid line indicates patients with infectious complications within 15 days postoperatively. An error bar shows the standard error of the mean. (Microsoft Excel for Macintosh, version 16.16.27, Microsoft). Tables
 Table 1. Patient characteristics.
Table 1. Patient characteristics. Table 2. Types of infectious complications.
Table 2. Types of infectious complications. Table 3. Comparison of changes in presepsin, procalcitonin, and C-reactive protein levels and leukocyte count between the infection and non-infection groups.
Table 3. Comparison of changes in presepsin, procalcitonin, and C-reactive protein levels and leukocyte count between the infection and non-infection groups. Table 4. Diagnostic accuracy of presepsin, procalcitonin, and CRP levels and leukocyte counts in terms of infectious complications on POD 5 and 7.
Table 4. Diagnostic accuracy of presepsin, procalcitonin, and CRP levels and leukocyte counts in terms of infectious complications on POD 5 and 7. Table 5. Comparison of patient characteristics between high and low preoperative levels of presepsin.
Table 5. Comparison of patient characteristics between high and low preoperative levels of presepsin. Table 1. Patient characteristics.
Table 1. Patient characteristics. Table 2. Types of infectious complications.
Table 2. Types of infectious complications. Table 3. Comparison of changes in presepsin, procalcitonin, and C-reactive protein levels and leukocyte count between the infection and non-infection groups.
Table 3. Comparison of changes in presepsin, procalcitonin, and C-reactive protein levels and leukocyte count between the infection and non-infection groups. Table 4. Diagnostic accuracy of presepsin, procalcitonin, and CRP levels and leukocyte counts in terms of infectious complications on POD 5 and 7.
Table 4. Diagnostic accuracy of presepsin, procalcitonin, and CRP levels and leukocyte counts in terms of infectious complications on POD 5 and 7. Table 5. Comparison of patient characteristics between high and low preoperative levels of presepsin.
Table 5. Comparison of patient characteristics between high and low preoperative levels of presepsin. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








