Latest Articles

23 Apr 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighting the Role of Advanced Practice Providers (Nurse Practitioners and Physician Assistants)
Willa V. Cochran, M. Veronica Dioverti
DOI: 10.12659/AOT.941185
Ann Transplant 2024; 29:e941185
23 Apr 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighting the Role of Advanced Practice Providers (Nurse Practitioners and Physician Assistants)
Willa V. Cochran, M. Veronica Dioverti
DOI: 10.12659/AOT.941185
Ann Transplant 2024; 29:e941185
16 Apr 2024 : Case report
Treatment of Cavernous Transformation of Portal Vein Caused by Hepatic Cystic Echinococcosis Using Ex Vivo Liver Resection and Autotransplantation
Ayidu Reyimu, Jiang Tiemin
DOI: 10.12659/AOT.942358
Ann Transplant 2024; 29:e942358
16 Apr 2024 : Case report
Treatment of Cavernous Transformation of Portal Vein Caused by Hepatic Cystic Echinococcosis Using Ex Vivo Liver Resection and Autotransplantation
Ayidu Reyimu, Jiang Tiemin
DOI: 10.12659/AOT.942358
Ann Transplant 2024; 29:e942358
09 Apr 2024 : Original article
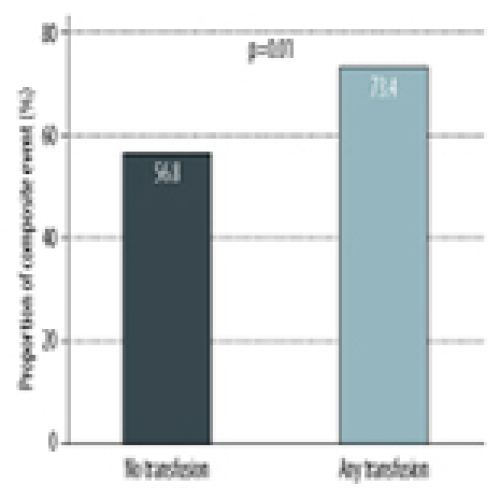
Impact of Blood Products Transfusion on Patients in the Immediate Post-Lung Transplant Period: A Cohort Study
Atif S. Siddiqui, Jawairia Shakil ![]()
DOI: 10.12659/AOT.943652
Ann Transplant 2024; 29:e943652
09 Apr 2024 : Original article
Impact of Blood Products Transfusion on Patients in the Immediate Post-Lung Transplant Period: A Cohort Study
Atif S. Siddiqui, Jawairia Shakil ![]()
DOI: 10.12659/AOT.943652
Ann Transplant 2024; 29:e943652
02 Apr 2024 : Original article
Association of Coronary Calcium Score on Cardiac PET During Pre-Kidney Transplant Assessment with Persistent Hyperparathyroidism: A Retrospective Study
Ziad Arabi, Mazin Ibrahim Musab El Sarrag
DOI: 10.12659/AOT.943532
Ann Transplant 2024; 29:e943532
02 Apr 2024 : Original article
Association of Coronary Calcium Score on Cardiac PET During Pre-Kidney Transplant Assessment with Persistent Hyperparathyroidism: A Retrospective Study
Ziad Arabi, Mazin Ibrahim Musab El Sarrag
DOI: 10.12659/AOT.943532
Ann Transplant 2024; 29:e943532
26 Mar 2024 : Original article
Outcomes of Renal Transplantation in ANCA-Associated Vasculitis
Xiaoqin Long, Xiaobing Yang
DOI: 10.12659/AOT.943433
Ann Transplant 2024; 29:e943433
26 Mar 2024 : Original article
Outcomes of Renal Transplantation in ANCA-Associated Vasculitis
Xiaoqin Long, Xiaobing Yang
DOI: 10.12659/AOT.943433
Ann Transplant 2024; 29:e943433
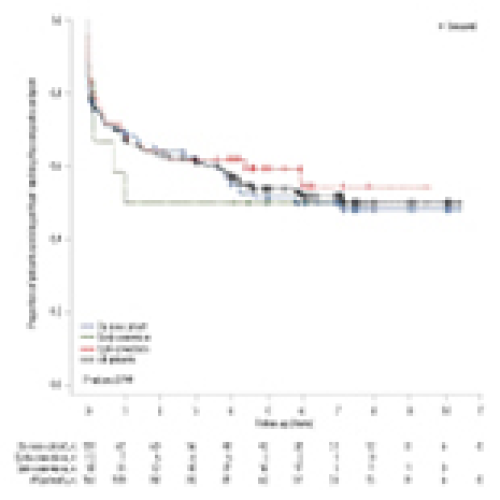
19 Mar 2024 : Original article
Long-Term Outcomes with Prolonged-Release Tacrolimus in Kidney Transplantation: A Retrospective Real-World Data Analysis
Wilfried Gwinner, Swapneel Anaokar
DOI: 10.12659/AOT.942167
Ann Transplant 2024; 29:e942167

19 Mar 2024 : Original article
Long-Term Outcomes with Prolonged-Release Tacrolimus in Kidney Transplantation: A Retrospective Real-World Data Analysis
Wilfried Gwinner, Swapneel Anaokar
DOI: 10.12659/AOT.942167
Ann Transplant 2024; 29:e942167
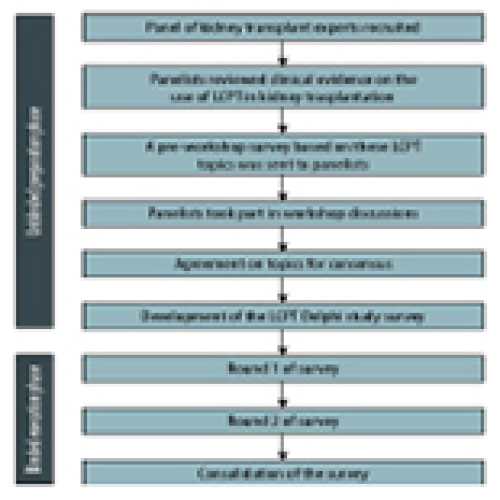
12 Mar 2024 : Original article
Use of LCP-Tacrolimus (LCPT) in Kidney Transplantation: A Delphi Consensus Survey of Expert Clinicians
Alexander Wiseman ![]() , Tarek Alhamad
, Tarek Alhamad ![]()
DOI: 10.12659/AOT.943498
Ann Transplant 2024; 29:e943498
12 Mar 2024 : Original article
Use of LCP-Tacrolimus (LCPT) in Kidney Transplantation: A Delphi Consensus Survey of Expert Clinicians
Alexander Wiseman ![]() , Tarek Alhamad
, Tarek Alhamad ![]()
DOI: 10.12659/AOT.943498
Ann Transplant 2024; 29:e943498
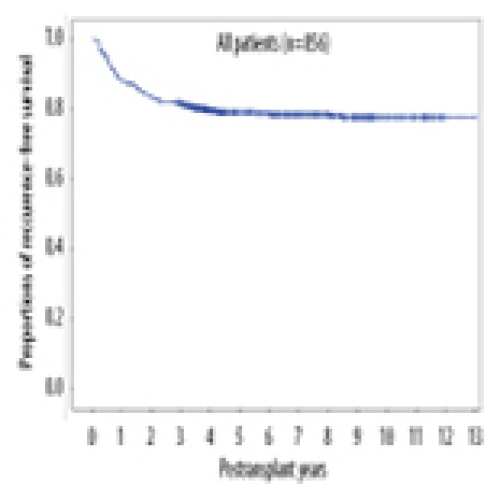
05 Mar 2024 : Original article
No Prognostic Impact of Graft-to-Recipient Weight Ratio on Hepatocellular Carcinoma Recurrence Following Living Donor Liver Transplantation
DOI: 10.12659/AOT.942767
Ann Transplant 2024; 29:e942767
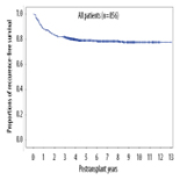
05 Mar 2024 : Original article
No Prognostic Impact of Graft-to-Recipient Weight Ratio on Hepatocellular Carcinoma Recurrence Following Living Donor Liver Transplantation
DOI: 10.12659/AOT.942767
Ann Transplant 2024; 29:e942767
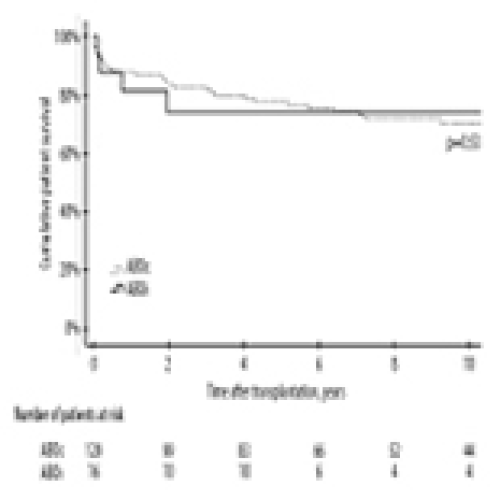
27 Feb 2024 : Case report
Successful Sequential Liver and Isolated Intestine Transplantation for Mitochondrial Neurogastrointestinal Encephalopathy Syndrome: A Case Report
Chandrashekhar A. Kubal ![]() , Plamen Mihaylov
, Plamen Mihaylov
DOI: 10.12659/AOT.941881
Ann Transplant 2024; 29:e941881
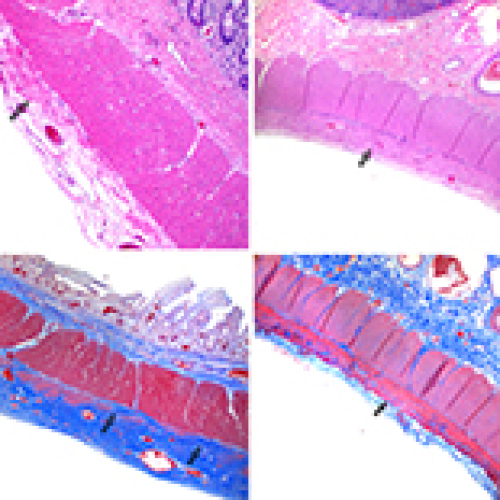
27 Feb 2024 : Case report
Successful Sequential Liver and Isolated Intestine Transplantation for Mitochondrial Neurogastrointestinal Encephalopathy Syndrome: A Case Report
Chandrashekhar A. Kubal ![]() , Plamen Mihaylov
, Plamen Mihaylov
DOI: 10.12659/AOT.941881
Ann Transplant 2024; 29:e941881

20 Feb 2024 : Original article
Lymphocele Outcomes After Renal Transplantations Performed by an Experienced Surgeon: Is Meticulously Performed Surgery and Experience Adequate to Prevent Lymphocele?
Nurettin Ay, Vahhac Alp
DOI: 10.12659/AOT.942656
Ann Transplant 2024; 29:e942656

20 Feb 2024 : Original article
Lymphocele Outcomes After Renal Transplantations Performed by an Experienced Surgeon: Is Meticulously Performed Surgery and Experience Adequate to Prevent Lymphocele?
Nurettin Ay, Vahhac Alp
DOI: 10.12659/AOT.942656
Ann Transplant 2024; 29:e942656
13 Feb 2024 : Original article
A Retrospective Study of Long-Term Outcomes in 16 ABO-Incompatible Deceased Donor Pediatric Liver Transplants from a National Transplant Center at Helsinki University Hospital, Finland, 1987-2022
Timo Jahnukainen ![]() , Inna Sareneva
, Inna Sareneva
DOI: 10.12659/AOT.941929
Ann Transplant 2024; 29:e941929
13 Feb 2024 : Original article
A Retrospective Study of Long-Term Outcomes in 16 ABO-Incompatible Deceased Donor Pediatric Liver Transplants from a National Transplant Center at Helsinki University Hospital, Finland, 1987-2022
Timo Jahnukainen ![]() , Inna Sareneva
, Inna Sareneva
DOI: 10.12659/AOT.941929
Ann Transplant 2024; 29:e941929
06 Feb 2024 : Original article
Immunoprotective Effect of Liver Allograft on Patients with Combined Liver and Kidney Transplantation
DOI: 10.12659/AOT.942763
Ann Transplant 2024; 29:e942763
06 Feb 2024 : Original article
Immunoprotective Effect of Liver Allograft on Patients with Combined Liver and Kidney Transplantation
DOI: 10.12659/AOT.942763
Ann Transplant 2024; 29:e942763
30 Jan 2024 : Original article
Cyclosporine A Does Not Mitigate Liver Ischemia/Reperfusion Injury in an Ex Vivo Porcine Model of Donation After Circulatory Death
DOI: 10.12659/AOT.941054
Ann Transplant 2024; 29:e941054
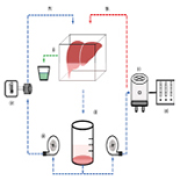
30 Jan 2024 : Original article
Cyclosporine A Does Not Mitigate Liver Ischemia/Reperfusion Injury in an Ex Vivo Porcine Model of Donation After Circulatory Death
DOI: 10.12659/AOT.941054
Ann Transplant 2024; 29:e941054
In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








